In vitro Antagonistic Activity of Candida albicans against Filamentous Fungi
Ali Abdul Hussein S AL Janabi, Duaa Ali, Noor A Mohammed and Fadhil Rahem
DOI10.21767/2471-8521.100002
Ali Abdul Hussein S AL Janabi*, Duaa Ali, Noor A Mohammed and Fadhil Rahem
Department of Clinical laboratories, Collage of Applied Medical Sciences, University of Karbala, Iraq
- *Corresponding Author:
- Ali Abdul Hussein S AL Janabi
Department of Clinical laboratories
Collage of Applied Medical Sciences
University of Karbala-Iraq
Tel: +964 32 321 364
E-mail: aljanabi_bio@yahoo.com.
Received date: October 17, 2015; Accepted date: October 27, 2015; Published date: November 26, 2015
Citation: AL Janabi AAHS, et al. In vitro Antagonistic Activity of Candida albicans against Filamentous Fungi. Med Mycol Open Access. 2015, 1:2. doi: 10.21767/2471-8521.100002
Copyright: © 2015 AL Janabi AAHS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Candida albicans is a special type of yeast that is naturally found on the surface of various mucosal layers of the human body. Biofilms forming is well known synergistic relationship between C. albicans and various organisms with no or little aggressive acted toward each other. Methods and findings: C. albicans that isolated from patient with cutaneous candidiasis was tested for antifungal activity against six species of filamentous fungi by measuring of percentage inhibition. The effects of incubation periods and different concentrations of glucose on the antifungal activity of C. albicans were also tested. The percentage inhibition of growing filamentous fungi had been observed at high values when they cultivated on media that previously cultured with C. albicans than when both of fungi and C. albicans cultured at the same time, especially at low concentrations of glucose. Mucor spp. and C. sitophila had been affected by C. albicans only when they cultivated on media that previously cultured with C. albicans. Conclusion: C. albicans had been shown the ability to inhibit various species of filamentous fungi. Incubation periods and glucose concentrations had been effected on the inhibitory action of C. albicans against other fungi.
Keywords
C. albicans; Filamentous fungi; Antifungal
Introduction
Candida albicans considers one of the most important fungi that is found in commensal with other normal microflora on mucosal surface of the mouth, esophagus, gastrointestinal tract, urogenital tract and on the skin of healthy individuals [1-3]. It is rarely found in the environment outside human body [4]. Under special conditions, C. albicans can be turned from unharmful yeast to pathogenic fungi causing many clinical forms of diseases that are called Candidiasis [1,5]. Some of these diseases located on superficial layers such as Cutaneous and vulvovaginial Candidiasis, while other invaded with dissemination into other tissues such as candidemia and candidial myocarditis [2,6]. Morphological alteration from yeast to pseudohyphae or hyphae and production of various virulence factors facilitate C. albicans to penetrate the mucosal layer and to cause systemic infections [1,6-7].
One of very important types of relationship between C. albicans and other normal microflora in the human body, especially fungi and bacteria, is the ability of C. albicans to form biofilms with the same specie or with different types of organisms that are found on the tissue surfaces through the production of tyrosol molecules [8]. These biofilms are more likely to hold C. albicans with heterogeneous organisms such as bacteria and non-candida albicans candidia species (NCAC) than with other strains of C. albicans [9-10].
Although C. albicans living in a synergistic form with different organism, it is also has the ability to eliminate other organism. The in vitro antagonistic ability of C. albicans toward other groups of fungi was investigated as the main aim in this study.
Materials and Methods
Fungal isolation
C. albicans was isolated from the skin of patient with Cutaneous Candidiasis who attended at AL-Hussein general teaching hospital of Karbala province in July 2014. In addition to germ tube and morphological characters, API 20C sugar assimilation system was performed to diagnosis of C. albicans [11-12]. Chrysonilia sitophila and four species of Aspergillus including A. flavus, A. fumigates, A. niger, and A. oryzae were isolated from air by exposure a plate of Sabouraud's dextrose agar (Himedia, India) to air for 10 min. Mucor spp. was isolated from contaminated bread. The isolated species of filamentous fungi were diagnosed based on morphological and microscopically characters [11,13], in addition to molecular characters that had been described by Gautam and Bhadauria [13]. All isolated fungi had been maintained on Sabouraud's dextrose agar as stock cultures.
Fungal and media preparation
The cell number of C. albicans and other isolated fungi had been standardized at 3 × 108 cells/ml by using McFarland Nephelometer barium sulfate standards [14]. Sabouraud's glucose agar was prepared by mixing peptone 10 g, agar 15 g, 1 L of distilled water and different concentrations of glucose (10 g, 15 g, 20 g, 25 g and 30 g). Those media were prepared in order to study the effect of glucose levels on the culturing of C. albicans with filamentous fungi.
Antifungal assay
The antagonistic relationship between C. albicans and other filamentous fungi was evaluated according to the method that mentioned by Randhawa et al. [15]. Briefly, C. albicans was cultivated like arc or half of the circle by swab around the center of culture plate that inoculated with 0.01 ml of filamentous fungi. The protocol was performed in two ways that differ in the time of inoculation of filamentous fungi with C. albicans. Firstly, C. albicans and other fungi were cultivated at the same time and incubation at 37°C for 48 h. Secondly, C. albicans was first incubated alone for 24 h at 37°C before inoculation of filamentous fungi, then incubated together at 37°C for 48 h. Meanwhile, each of C. albicans and other fungal species were separately cultured on prepared media as a control. Perpendicular colony diameters (mm) of growing species were measured and percentage inhibition was calculated according to the formula that mentioned by Kumar et al. [16] as follow:
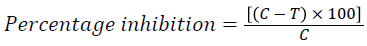
Where, C=colony diameter (mm) of control
T=colony diameter (mm) of tested fungi
Results
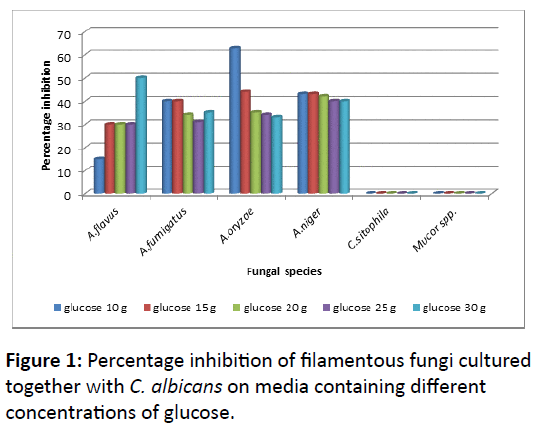
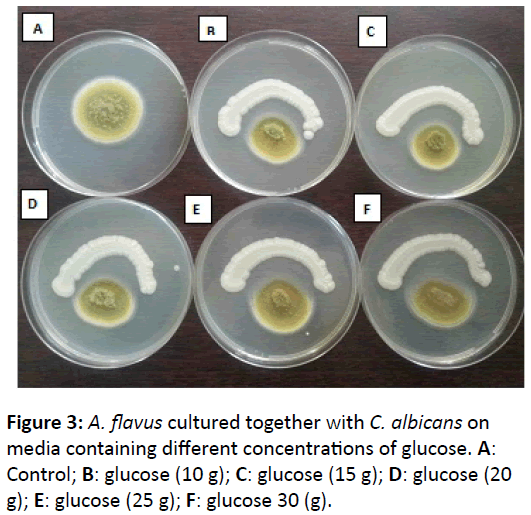
The ability of C. albicans to inhibit the growth of other fungi had been investigated against six species of filamentous fungi. After 48 h incubation of testing fungi with C. albicans, the growth of A. niger, followed by A. oryzae and A. fumigatus had been ceased with high percentage inhibition, especially at low concentrations of glucose (10 g). However, the inhibition rate of those three species was decreased in concomitant with increasing of glucose concentrations. For A. flavus, the result was reflected when fungal growth decreased on media containing low concentrations of glucose (Figure 3). On the other hand, the growth of Mucor spp. and C. sitophila had not been effected in the presence of C. albicans even glucose concentration was affected (Figure 1).
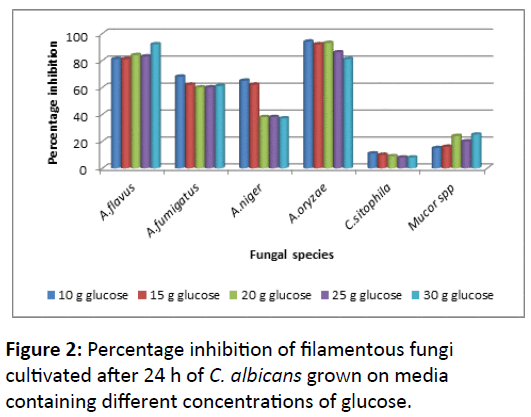
The growth of filamentous fungi that had been inoculated after 24 h cultivation of C. albicans was clearly decreased with high percentage inhibition than when fungi and C. albicans cultured together at the same time. A. oryzae followed by A. fumigatus and A. niger had been shown more sensitivity toward the growth of C. albicans with decreasing in inhibition values in concomitant with decreasing of glucose concentrations (Figure 2).
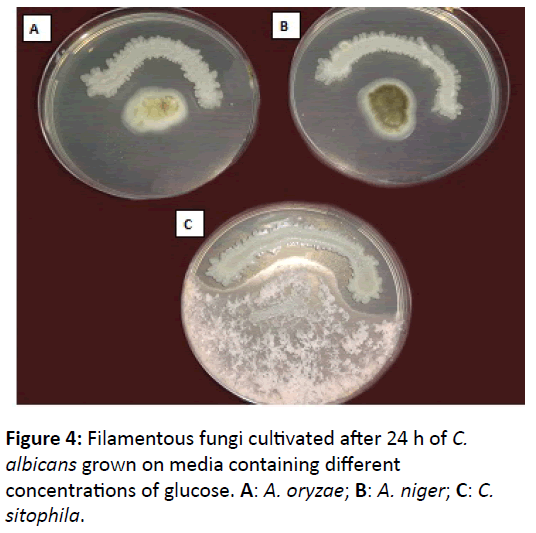
In the second method, the growth of Mucor species and C. sitophila was differed from that in the first method. Both of them had been affected by the culturing of C. albicans, especially at low concentrations of glucose for C. sitophila and high concentrations for Mucor species (Figure 4).
Discussion
C. albicans that is found on mucosal surface of different organs of the human body is usually cannot live alone. It represented one of various groups of organisms that are living together in the form of microflora. Although C. albicans can not cause any harmful effects on the healthy individuals body, but in special conditions such as a deficiency in the immune system, it can become pathogenic fungi and have the ability to cause various forms of Candidiasis [1,5].
The relationship between C. albicans and other fungi does not consider in peace all of time as in forming of biofilms. It can be aggressive relationship when C. albicans tends to eliminate other fungi as noted for A. fumigatus [15]. This inhibitory activity of C. albicans may be related to its ability to produce suppressive materials that are effecting on the growth of other fungi. In human body, C. albicans usually secreted various hydrolytic enzymes such as aspartyl proteases, phospholipase A to D, serine peptidase and at least nine lipases [2-3,17-20]. It is also producing gliotoxin [21-24] which is an epipolythiodioxoperazine with a molecular mass of 326 Da that mainly produced by A. fumigatus [25-28]. However, hydrolytic enzymes and gliotoxin consider an important virulence factors for the pathogenicity of C. albicans [1,5].
C. albicans that was cultivated with filamentous fungi on media containing different concentrations of glucose had been revealed the ability to inhibit the growth of other tested fungi. Although we didn't identify the types or nature of the substances that had secreted by C. albicans into culture media and had showed antifungal effects, gliotoxin that is encoded by the gli gene cluster as detected in A. fumigatus [29] is assumed to be one of them due to its known toxicity against various groups of fungi [30]. In mammalian cells, the toxicity of gliotoxin is related to the presence of disulphide bridge in its structure [28,31]. Whereas, in fungal cells, gliotoxin has often interfered with the activity of redox homeostasis or protein modification leading to a disorder of the cell function and growth [29]. Otherwise, gliotoxin have the ability to inhibit the function of acetolactate synthase that is important to produce acetaldehyde in fungal cells [26,32].
Filamentous fungi had been shown a variable degree of sensitivity toward the presence of C. albicans and that may be depended on the type and amounts of secreted materials from growing yeast. The colony of A. niger had been clearly inhibited in the presence of C. albicans when they were cultured at the same time, while it inhibited less than A. oryzae and A. fumigatus in plated that previously cultured with C. albicans. The synthesis of gliotoxin in fungal cells is usually needed a long period of incubation time due to its produced in the late stage of growth as secondary metabolites [28]. Thus, delay incubation of C. albicans for 24 h may give the yeast enough time to increase its secretion of gliotoxin in the media and that would be elevated the inhibitory action of it against tested filamentous fungi. This explanation could be confirmed by the results of Carberry et al. who found that A niger was inhibited at low concentration of gliotoxin (5 μg/ml) than A. flavus and A. oryzae which they need a high concentration of gliotoxin (10 μg/ml) to inhibit [30]. This observation was also cleared with Mucor spp. and C. sitophila in our study. They hadn't affected when they cultured together with C. albicans, while their growth had been inhibited on media that previously cultured with C. albicans.
In addition to incubation periods, temperature could be considered another factor that effects on the secretion of gliotoxin. Incubation at 37°C found to be increased the production of gliotoxin by A. fumigatus than at 25°C due to the role of high temperature in inducing fast growing of fungal hyphae [28].
A. fumigatus is one of important gliotoxin producing fungi. It has a specific self-protection system against the effect of gliotoxin [29], unless it loses one of gliotoxin synthesis genes that rendered the fungus more sensitive to exogenous gliotoxin [33-34]. Thus, to explain the sensitivity of A. fumigatus toward the cultured of C. albicans in our study, there must be other substances produced by C. albicans in addition to gliotoxin. Glucose is the main source of energy for all living organisms including fungi. In C. albicans, glucose is usually catalytic through various metabolic pathways to get energy and to produce many useful chemical compounds for its survival, such as ethanol [35]. Ethanol that considers germistatic fungal agent has the ability to inhibit the growth of a wide range of fungal species [36]. Its production by C. albicans had been found to be increased at 37°C than at low temperatures [37] and because we was incubated C. albicans with other fungi at 37°C , so we were assuming that ethanol was produced in amounts enough to inhibit the growth of filamentous fungi. Moreover, C. albicans can also produce CO2 from the metabolic pathway of glucose. In the presence of 25% CO2, the growth of many fungi had been reduced [38], while A. niger had been inhibited at 3% CO2 [39,40].
For most of tested fungi, the percentage inhibition had been affected by glucose concentrations. At low concentrations of glucose, the growth rate was very clearly decreased than at high concentrations. Thus, if we assume that C. albicans needs a short time to convert glucose to ethanol when it is in small amounts, then greater quantity of glucose often takes a long time to break down. However, the high percentage inhibition that had been shown in plates with previously cultured of C. albicans may result from the presence of many secondary metabolites such as gliotoxin and ethanol.
The confirmation of the inhibitory action of C. albicans against other fungi is not just made C. albicans more competent fungi among other normal flora, but it also gives the human body, another line of defense against opportunistic fungi. The main route of entering of fungi into the human body is usually through the respiratory system. C albicans is usually found on the mucosal surface of the nose and mouth [1-2]. Thus, entering of fungal cells into human body by inhalation and deposit most of them on the mucosal surface will follow by the destruction of these cells with no chance to grow due to the presence of C. albicans and its inhibitory secretions. Thus, in vivo study is recommended to confirm the capacity of C. albicans to eliminate filamentous fungi from the human body.
Conclusion
C. albicans had been shown the ability to inhibit various species of filamentous fungi. Incubation period and glucose concentration had been effected on the inhibitory action of C. albicans against other fungi. The human body will get another advantage from the presence of C. albicans as normal flora through its ability to eliminate other opportunistic fungi.
References
- Nasution AI (2013) Virulence factor and pathogenicity of Candida albicans in oral Candidiasis. World J Dentistry 4: 267-271.
- Tsai PW, Chen YT, Hsu PC, Lan CY (2013) Study of Candida albicans and its interactions with the host: a mini review. BioMedicine 3: 51-64.
- Mardegan RC, Foglio MA, Gon?alves RB, Höfling JF (2006) Candida albicansproteniases. Braz J Oral Sci J 5: 944-952.
- Hiller E, Zavrel M, Hauser N, Sohn K, Kentischer AB, et al. (2011) Adaptation, adhesion and invasion during interaction of Candida albicans with the host-focus on the function of cell wall proteins. Int J Med Microbiol 301: 384-389.
- Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4: 119-128.
- Molero G, Orejas RD, García FN, Monteoliva L, Pla J, et al. (1998) Candida albicans: genetics, dimorphism and pathogenicity. InternatlMicrobiol 1: 95-106.
- Sudbery P, Gow N, Berman J (2004) The distinct morphogenic states of Candida albicans. Trends in Microbiology 12: 317-324.
- Alem MAS, Oteef MDY, Flowers TH, Douglas LJ (2006) Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilms development. Eukaryotic Cell 5: 1770-1779.
- EI-Azizi MA, Starks SE, Khardori N (2004) Interactions of Candida albicans with other Candida spp. and bacteria in the biofilms. J Applied Microbiology 96: 1067-1073.
- Pathak AK, Sharma S, Shrivastva P (2012) Multi-species biofilms of Candida albicans and non-Candida albicans Candida species on acrylic substrate. J Appl Oral Sci 20: 70-75.
- Ellis D, Davis S, Alexiou H, Handke R, Bartley R (2007) Descriptions of medical fungi. (2ndedn.) Mycology unit women's and children's hospital. Australia.
- Bhavan PS, Rajkumar R, Radhakrishnan S, Seenivasan C, Kannan S (2010) Culture and identification of Candida albicans from vaginal ulcer and separation of enolase on SDS-PAGE. International J Biology 2: 84-93.
- Gautam AK, Bhadauria R (2012) Characterization of Aspergillus species associated with commercially stored triphala powder. African J Biotechnology 11: 16814-16823.
- Brown AE (2012) Bensons microbiological applications: laboratory manual in general microbiology complete version. (12thedn.) McGraw-Hill international edition.
- Randhawa HS, Sandhu RS, Kowshik T (2002) In vitro inhibition of Aspergillusfumigatus by Candida species, especially C. albicans and C. glabrata. Current Science 82: 860-865.
- Kumar P, Dhiman S, Bhatt RP, Singh L (2011) In-vitro antifugal activity of Sapiumsebiferum L. against Aspergillusniger and aflatoxigenicAspergillusflavus. J Applied Pharmaceutical Science 1: 108-110.
- Aka?lar S, Ener B, Tore O (2011) Acid proteinase enzyme activity in Candida albicans strains: a comparison of spectrophotometry and plate methods. Turk J Biol 35: 559-567.
- Mahmoudabadi AZ, Zarrin M, Miry S (2010) Phospholipase activity of Candida albicans isolated from vagina and urine samples. JJM 3: 169-173.
- Tsuboi R, Komatsuzaki H, Ogawa H (1996) Induction of an extracellular esterase from Candida albicans and some of its properties. Infect Immun 64: 2936-2940.
- Meurman JH, Siikala E, Richardson M, Rautemaa R (2007) Non-Candida albicans Candida yeasts of the oral cavity.A Mendez-Vilas (ed.) Communicating Current Research and Educational Topics and Trends in Applied Microbiology 1: 719-731.
- Bertling A, Niemann S, Uekötter A, Fegeler W, Lass-Flörl C, et al. (2010) Candida albicans and its metabolite gliotoxin inhibit platelet function via interaction with thiols. ThromHaemost 104: 270-278.
- Taha M (2001) The production of the mycotoxin (gliotoxin) by Candida albicans in patients with oral Candidasis. Egyptian J Dermatology and Anderology 21: 21-26.
- Shah DT, Glover DD, Larsen B (1995) In situ mycotoxin production by Candida albicans in women with vaginitis. GynecolObstet Invest 39: 67-69.
- Shah DT, Larsen B (1991) Clinical isolates of yeast produce a gliotoxin-like substance. Mycopathologia 116: 203-208.
- Sutton P, Newcombe NR, Waring P, Mullbacher A (1994) In vitro immunosuppressive activity of gliotoxin, a metabolite produced by human pathogenic fungi. Infection and Immunity 62: 1192-1198.
- McCourt JA, Duggleby RG (2006) Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 31: 173-210.
- Gardiner DM, Waring P, Howlett BJ (2005) The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology 151: 1021-1032.
- Kosalc I, Pepeljnjak S, Jandrli M (2004) Influence of media and temperature on gliotoxin production in Aspergillusfumigatus strains. 3rd Croatian Congress of Toxicology : 26-29
- Dolan SK, OKeeffe G, Jones GW, Doyle S (2015) Resistance is not futile: gliotoxin biosynthesis, functionally and utility. Trends Microbiol 23: 419-428.
- Carberry S, Molloy E, Hammel S, OKeeffe G, Jones GW, et al. (2012) Gliotoxin effects on fungal growth: Mechanisms and exploitation. Fungal Genetics and Biology 49: 302-312.
- Jones RW, Hancock JG (1988) Mechanism of gliotoxin action and factors mediating gliotoxin sensitivity. J General Microbiology 134: 2067-2075.
- Haraguchi H, Yamano K, Kusunoki N, Fukuda A (1997) Effect of gliotoxin and related compounds on acetolactate synthase. J Agric Food Chem 45: 2784-2787.
- Schrettl M, Carberry S, Kavanagh K, Haas H, Jones GW, et al. (2010) Self-protection against gliotoxin- a component of the gliotoxin biosynthetic cluster. GliT, completely protects Aspergillusfumigatus against exogenous gliotoxin. PLoS Pathogens 6: 1-15.
- Chamilos G, Lewis RE, Lamaris GA, Albert ND, Kontoyiannis DP (2008) Genomewide screening for genes associated with gliotoxin resistance and sensitivity in Saccharomyces cerevisiae. Antimicrobial Agents and Chemotherapy 52: 1325-1329.
- Lough PS, Fehn R (1993) Efficacy of 1% sodium fluoride as a preservative in urine samples containing glucose and Candida albicans. J Forensic Sci 38: 266-271.
- Utama IM, Wills RB, Ben-Yehoshua S, Kuek C (2002) In vitro efficacy of plant volatiles for inhibiting the growth of fruit and vegetable decay microorganisms. J Agric Food Chem 50: 6371-6377.
- Chang J, Kollman SE (1989) The effect of temperature on the formation of ethanol by Candida albicans in blood. J Forensic Sciences 34: 105-109.
- Giorni P, Battilani P, Pietri A, Magan N (2008) Effect of aw and CO2 level on Aspergillusflavus growth and aflatoxin production in high moisture maize postharvest. In j Food Microbiol 122: 109-113.
- Mclntyre M, McNeil B (1997) Dissolved carbon dioxide effects on morphology, growth, and citrate production in Aspergillusniger A60. Enzyme and Microbial Technology 20: 135-142.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences