Aspergillus flavus Disseminated Infection in Paediatric Acute Lymphoblastic Leukaemia: A Case Report
Elena Varotto, Maria Caterina Putti, Dino Sgarabotto, Diego Cecchin, Federica De Corti, Valeria
Beltrame, Giada Biddeci, Maria Paola Boaro, Pietro Zucchetta, Giuseppe Basso and Barbara
Buldini
DOI10.21767/2471-8521.100020
Elena Varotto1, Maria Caterina Putti1, Dino Sgarabotto2, Diego Cecchin3, Federica De Corti4, Valeria Beltrame5, Giada Biddeci1, Maria Paola Boaro1, Pietro Zucchetta3, Giuseppe Basso1 and Barbara Buldini1*
1Paediatric Heamato-Oncology Clinic, Department of Woman and Child Health, University of Padova, Padova, Italy
2Tropical and Infectious Diseases Unit, Padova University Hospital, Padova, Italy
3Nuclear Medicine Unit, Department of Medicine DIMED, University-Hospital of Padova, Italy
4Pediatric Surgery Unit, Department of Woman and Child Health, University-Hospital of Padova, Padova, Italy
5Department of Medicine, University Hospital of Padua, University Radiology, Padova, Italy
- Corresponding Author:
- Barbara Buldini
Paediatric Heamato-Oncology Clinic, Department of Woman and Child Health
University of Padova, Padova, Italy
Tel: 0498211457
E-mail: barbarabuldini@hotmail.com
Received Date: October 21, 2016; Accepted Date: November 24, 2016; Published Date: December 04, 2016
Citation: Varotto E, Caterina Putti M, Sgarabotto D, Cecchin D, De Corti F, et al. (2016) Aspergillus flavus Disseminated Infection in Paediatric Acute Lymphoblastic Leukaemia: A Case Report. Med Mycol Open Access 2:5. doi: 10.21767/2471-8521.100020.
Copyright: © 2016 Varotto E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Here we report the case of a disseminated Aspergillus flavus infection in an adolescent affected by acute lymphoblastic leukaemia at the beginning of first-line chemotherapy. Association of surgery and combination antifungal therapy (high dose liposomal amphotericin B and caspofungin) allowed infection improvement. 18-FDG PET-MRI was used for response-to therapy monitoring.
Abstract
Here we report the case of a disseminated Aspergillus flavus infection in an adolescent affected by acute lymphoblastic leukaemia at the beginning of first-line chemotherapy. Association of surgery and combination antifungal therapy (high dose liposomal amphotericin B and caspofungin) allowed infection improvement. 18-FDG PET-MRI was used for response-to therapy monitoring.
Introduction
Incidence of invasive fungal infections (IFI) has progressively increased over the past few decades [1]. Invasive aspergillosis (IA) is associated with the highest morbidity and mortality in immunocompromised patients [2,3]. Aspergillus flavus is the second leading cause of IA affecting mostly upper airways and skin [4,5]. Invasive cutaneous asperigillosis (ICA) is a rare condition characterized by more or less itching macules, papules, plaques or haemorrhagic bullae, potentially evolving into necrotic ulcers covered by a dark eschar. Primary ICA, deriving from fungus direct inoculation into an injured site, can be distinguished from secondary ICA, usually resulting from systemic dissemination of inhaled hyphae through the blood stream [6-10]. Immunological deficiency in paediatric haemato-oncology patients is due both to the malignancy and chemotherapy regimen, emerging as the main predisposing factors to IFI [11]. Early diagnosis, effective therapy and accurate response-to-therapy monitoring are mandatory in these patients, in order to achieve infection control and reintroduce chemotherapy as soon as possible.
Here we report the case of a proven invasive Aspergillus flavus infection in an adolescent affected by acute lymphoblastic leukaemia (ALL), with particular attention to clinical presentation, therapy schedule and response-to-therapy monitoring.
Description
A 17 years old girl followed at Paediatric Oncology- Haematology Clinic of Padova for B-precursor ALL developed a progressive painful swelling of the left forearm. Diagnosis of B-III ALL according to EGIL classification [12] was done in August 2016; central nervous system involvement was excluded. Soon after the diagnosis, the patient was enrolled in AIEOP BFM ALL 2009 international protocol. After the end of the first chemotherapeutic phase Induction A (prednisone 60 mg/m2/day for 28 days; vincristine 1.5 mg/m2 weekly for 4 doses; daunorubicin 30 mg/m2 weekly for 4 doses; PEG-asparaginase 2500 UI/m2 for 2 doses every 2 weeks) the patient developed a painful swelling of the left forearm without alteration of overlying skin. According to personal history of a recent accidental trauma and ultrasound findings, the lesion was initially interpreted as a muscular haematoma. When fever appeared, the girl was hospitalized and wide spectrum antibiotic therapy was started (ceftazidime 40 mg/kg/day TID, amikacin 15 mg/kg QD; teicoplanin 10 mg/kg BID the first day, then 10 mg/kg QD), by considering concomitant severe neutropenia and possible haematoma superinfection. For the persistence of fever longer than 72 h, according to our local protocol, antibiotic therapy was shifted to meropenem and antifungal therapy (liposomial amphotericin B 3 mg/kg QD) was started. During hospitalization we observed a worsening of the cutaneous lesions: the first one evolved into a hyperemic painful nodule; three analogue new lesions appeared on the left leg and left arm, one of which covered by a haemorrhagic bullae (Figure 1).
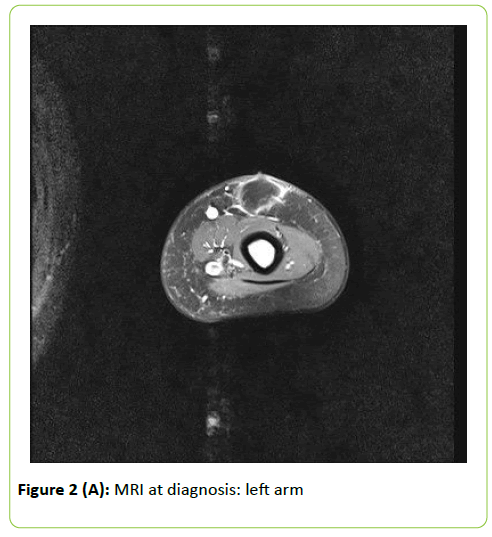
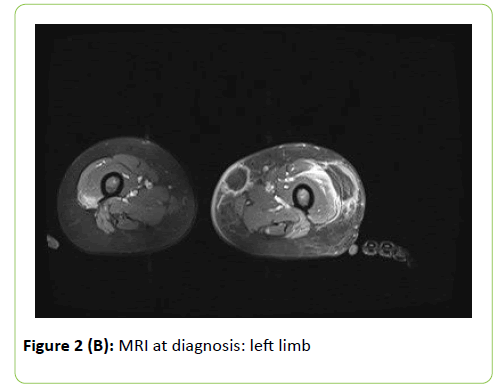
Inflammation indexes were only mildly elevated (maximum Creactive protein value: 35 mg/L). Initial Galactomannan (GM) and 1-3,Beta-D-Glucan (BG) serum dosages were negative. Ultrasound evaluation and local Magnetic Resonance Imaging (MRI) showed subcutaneous colliquating abscesses (Figure 2). Surgical drainage was performed and abundant purulent material collected: Aspergillus flavus was isolated. According to previous experience at our Centre in fungal infection diagnosing and monitoring, a 18-fluorodeoxyglucose Positron Emission Tomography-MRI (18-FDG PET-MRI)) was performed. This exam allows to study anatomically and functionally the whole body, detecting disseminated lesions and minimizing radiation exposure [13]. Mild to high metabolic uptake (Standardized Uptake Value–SUV 2.4-4.8) was shown at the following sites: right leg (2 muscular lesions); left leg (2 subcutaneous lesions); right dorsal muscles (1 lesion); left arm (2 subcutaneous lesions); left underarm (1 lymphatic lesion); kidneys (2 parenchymal lesions); right upper pulmonary lobe (1 lesion). No central nervous system (CNS) involvement was detected. Eyes evaluation and echocardiography excluded respectively ocular and cardiac involvement.
Combination antifungal therapy with high dose liposomal amphotericin B (7.5 mg/kg QD) and caspofungin (70 mg QD the first day, then 50 mg QD) was started. We opted for this intensive pharmacological schedule by considering the extension of fungal infection and the necessity to control it promptly and reintroduce chemotherapy as soon as possible. Surgical curettage of subcutaneous abscesses was performed three times a week by paediatric surgeons. We observed fever resolution after 3 days of combination therapy and slow improvement of subcutaneous lesions.
GM was persistently negative, while BG increased progressively (maximum value>523 pg/ml). After 21 days of combination therapy we repeated 18-FDG PET-MRI, that showed a partial improvement of the fungal lesions. This finding allowed us to start a maintenance chemotherapy with oral methotrexate and 6-mercaptopurine waiting for a complete resolution of the infection.
Discussion
Invasive aspergillosis has been emerging as an increasingly important cause of morbidity and mortality in immunocompromised children. Aspergillus fumigatus is the most frequent pathogen, followed by Aspergillus flavus and Aspergillus terreus [14,15]. IA usually develops soon after prolonged and severe neutropenia and commonly affects lungs, less often paranasal sinuses, central nervous system (CNS), skin and soft tissues. Disseminated form accounts for about 30% of cases [7,16]. Clinical presentation may be aspecific (i.e. prolonged fever in severe neutropenia not responding to wide spectrum antibiotic therapy) or as expression of organ damage (i.e. cough, pleural pain, and haemoptysis in lung infection or focal neurological deficits or seizures in CNS localization).
Primary cutaneous aspergillosis has been reported more frequently in children than in adults, in association with direct skin lacerations [16,17]. Our patient developed a disseminated Aspergillus flavus infection with multiple subcutaneous, muscular and parenchymal lesions. IA is an unexpected finding in paediatric ALL at the beginning of the first line therapy, as we observed in our patient: no antifungal prophylaxis is usually recommended in paediatric ALL [18-20]. Even if patient’s personal history was positive for a traumatic event, disseminated IA was more likely the consequence of fungal vascular migration from an initial pulmonary site than direct inoculation of Aspergillus flavus into the skin, also considering that no cutaneous laceration was detected or referred.
IA prognosis mainly depends on early diagnosis, appropriate treatment and restoration of host defences [21]. Timely infection control is mandatory in haemato-oncology paediatric patients, considering that a prolonged interruption of chemotherapy may affect dramatically the oncological prognosis. On the other hand IA diagnosis, therapy and monitoring are still challenging. Isolation of fungal pathogen is necessary for the definition of proven IA, but is rare and usually needs surgical approaches [22].
Galactomannan (GM), a polysaccharide cell-wall component of all Aspergillus spp, is the most accurate marker for IA screening in adults, but has shown a wide range of true-positive and true-negative results in paediatric studies (sensitivity 0.76; specificity 0.86) [23]. In our patient serum GM was repeatedly negative, even in presence of a proven Aspergillus flavus infection. 1-3, Beta-D-Glucan (BG) is a cell wall component of many fungal pathogens such as Aspergillus spp, Candida spp, Fusarium, Trichosporum or Saccharomyces, and Pneumocystis jiroveci, whereas lacks in Cryptococcus neoformans and Mucorales. Its serum testing has been proving to be useful in adult patients, but data are too limited in children to recommend [24]. In our patient basal serum BG was negative and increased progressively during therapy to the maximum detectable level (>523 pg/ml). In our opinion BG should be considered as an indicator of the presence or absence of fungal infection more than an indirect estimator of disease extension. The increasing serum levels in our patient might be due to moulds destruction rather than to IA progression.
As regards radiological investigation, CT and MRI have a high accuracy in the early diagnosis of IA, but may result inadequate in evaluating disseminated disease and monitoring of residual infection. Furthermore cumulative radiation exposure due to CT scan has to be considered, especially in paediatric population [25,26]. 18-FDG PET-MRI has been recently proposed as a combined functional-anatomical methodical to better distinguish active fungal lesions from residual scars [13]. In our patient 18- FDG PET-MRI allowed to study the whole body, detected additional lesions and monitored their evolution in terms of activity and anatomical definition.
No standardized therapy are available, both for the variability of local epidemiology and paucity of clinical trials [27]. ECIL-5 guidelines recommended monotherapy admistration in IA (voriconazole 2 x 6 mg/kg the first day, then 2 x 4 mg/kg - evidence grade AI - or liposomal amphotericin B 3 mg/kg – evidence grade BI) [18]. Anyway clinical experience seems to indicate combination therapy as an efficacious treatment of IA in heamato-oncology paediatric patients, in order to accelerate the infection control and reduce chemotherapy delay. Association of caspofungin and voriconazole or caspofungin and liposomal amphotericin B has been previously described [9,28-30]. Combination therapy with high dose liposomal amphotericin B (5-7.5 mg/kg) and caspofungin (70 mg the first day, then 50 mg/ day) was well tolerated in our patient. No renal or liver impairment was detected. Moderate hypokalemia appeared and was easily corrected with intravenous potassium chloride infusion. Surgical curettage of necrotic lesions had a leading role in the improvement of subcutaneous localizations.
In conclusion, IA is a challenging complication of chemotherapy-induced immunosuppression in paediatric haemato-oncology patients. It requires a multidisciplinary approach in order to obtain promptly the most appropriate diagnosis. Aggressive therapy is mandatory to control the infection and allows the clinician to reduce chemotherapy suspension. 18-FDG PET-MRI may be useful for a more accurate diagnosis and response-to-therapy monitoring.
References
- Dasbach EJ, Davies GM, Teutsch SM (2000) Burden of aspergillosis related hospitalizations in the United States. Clin Infect Dis 31:1524-1528.
- Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, et al. (2012)Clinical epidemiology of 960 patients with invasive aspergillosis fromthe PATH Alliance registry. J Infect 65:453-464.
- Beluffi G, Bernardo ME, Meloni G, Spinazzola A, Locatelli F (2008)Spinal osteomyelitis due to Aspergillusflavus in a child: a rarecomplication after haematopoietic stem cell transplantation. PediatrRadiol 38: 709-712.
- Hedayati M, Pasqualotto A, Warn P, Bowyer P, Denning D (2007)Aspergillusflavus: human pathogen, allergen and mycotoxin producer. Microbiology 153:1677-1692.
- Khodavaisy S, Badali H, Hashemi SJ, Aala F, Nazeri M, et al. (2016) Invitro activities of five antifungal agents against 199 clinical andenvironmental isolates of Aspergillusflavus, an opportunistic fungalpathogen. Journal de Mycologie Medicale 26: 116-121.
- van Burik JA, Colven R, Spach DH (1998) Cutaneous aspergillosis. JClinMicrobiol 36:3115-3121.
- Walmsley S, Devi S, King S, Schneider R, Richardson S, et al. (1993)Invasive Aspergillus infections in a pediatric hospital: A ten-yearreview. Pediatr Infect Dis J 12:673-682.
- Chakrabarti A, Gupta V, Biswas G, Kumar B, Sakhuja VK (1998)Primary cutaneous aspergillosis: Our experience in 10 years. J Infect 137: 24-27.
- Bernardeschi C, Foulet F, Ingen-Housz-Oro S, Ortonne N, Sitbon K, etal. (2015) Cutaneous invasiveaspergillosis: retrospective multicenter study of the French Invasive-Aspergillosis Registry and Literature Review. Medicine 94: e1018.
- Tak V, Mathur P, Xess I, Kale P, Sagar S, et al. (2013) A case of dualinfection in a paediatric trauma victim of primary cutaneousaspergillosis caused by Aspergillusflavus and Aspergillusterreus. Indian J Med Microbiol 31:193-196.
- Lehrnbecher T, Phillips R, Alexander S, Alvaro F, Carlesse F, et al. (2012) International Pediatric Fever and Neutropenia Guideline Panel.Guideline for the management of fever and neutropenia in children withcancer and/or undergoing hematopoietic stem-cell transplantation. JClin Oncol 30:4427-4438.
- Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, et al. (1995)Proposals for the immunological classification of acute leukemias.European Group for the Immunological Characterization of Leukemias(EGIL). Leukemia 9:1783-1786.
- Carraro S, Cecchin D, Sgarabotto D, Bozzetto S, Zucchetta P, et al. (2016) 18F-FDG PET/MRI for monitoring disseminated aspergillosis ina 16-year-old boy. Pediatric Infectious Disease 23.
- Zaoutis TE, Heydon K, Chu JH, Walsh TJ, Steinbach WJ (2006)Epidemiology, outcomes, and costs of invasive aspergillosis inimmunocompromised children in the United States, 2000. Pediatrics 117: 711-716.
- Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, et al. (2008) Pediatric invasive aspergillosis: a multicenter retrospectiveanalysis of 139 contemporary cases. Pediatrics 121: 1286-1294.
- Walsh T, Gonzalez C, Lyman C, Chanock S, Pizzo P (1996) Invasivefungal infections in children: recent advances in diagnosis andtreatment. Advances in Pediatr Infect Dis 11: 187-290.
- Groll AH, Schrey D, Tragiannidis A, Bochennek K, Lehrnbecher T(2013) Invasive aspergillosis in children and adolescents. Current Pharmaceutical Design 19: 3545-3568.
- Haerbrecht R, Tissot F, Agrawal S, Pagano L, Pettrikos G, et al. (2013)2013-Update of the ECIL guidelines for antifungal therapy in leukaemiaand HSCT patients (ECIL 5).
- Pagano L, Caira M, Picardi M, Candoni A, Melillo L, et al. (2007)Invasive Aspergillosis in patients with acute leukemia: update onmorbidity and mortality-SEIFEM-C. Report Clin Infect Dis 1:11.
- Maertens J, Donnely P, Kibbler K, Duarte R, Cornely O, et al. (2013)ECIL 5. Primary antifungal prophylaxis.
- Roilides E (2006) Early diagnosis of invasive aspergillosis in infantsand children. Med Mycol 44:199-205.
- De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE (2008)European Organization for Research and Treatment ofCancer/Invasive Fungal Infections Cooperative Group; NationalInstitute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Revised definitions of invasive fungal disease from theEuropean Organization for Research and Treatment ofCancer/Invasive Fungal Infections Cooperative Group and the NationalInstitute of Allergy and Infectious Diseases Mycoses Study Group(EORTC/MSG) Consensus Group. Clin Infect Dis 46: 1813-1821.
- Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, et al. (2014)Fourth European Conference on Infections in Leukaemia (ECIL-4):guidelines for diagnosis, prevention, and treatment of invasive fungaldiseases in paediatric patients with cancer or allogeneic haemopoieticstem-cell transplantation. Lancet Oncol 15: 327-340.
- Oz Y, Kiraz N (2011) Diagnostic methods for fungal infections inpediatric patients: microbiological, serological and molecular methods. Expert Rev Anti Infect Ther 9: 289-298.
- Hall EJ, Brenner DJ (2008) Cancer risks from diagnostic radiology. Br JRadiol81: 362-378.
- Robbins E (2008) Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer 51: 453-457.
- Morgan JE, Hassan H, Cockle JV, Lethaby C, James B, et al. (2016)Critical review of current clinical practice guidelines for antifungaltherapy in paediatrichaematology and oncology Support Care Cancer.
- Zhang M, Su X, Sun WK, Chen F, Xu XY, et al. (2014) Efficacy of the combination of Voriconazole and Caspofungin in experimentalpulmonary aspergillosis by different Aspergillus species.Mycopathologia177: 11-18.
- Cesaro S, Giacchino M, Locatelli F, Spiller M, Buldini B, et al. (2007)Safety and efficacy of a caspofungin-based combination therapy fortreatment of proven or probable aspergillosis in pediatric haematological patients. BMC Infect Dis18: 28.
- Elefanti A, Mouton JW, Verweij PE, Tsakris A, Zerva L, et al. (2103) Amphotericin B- and Voriconazole-Echinocandin combinations againstAspergillusspp: effect of serum on inhibitory and fungicidalinteractions. Antimicrob Agents Chemother 57:4656-4663.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences