Bioactive Fungal Endoperoxides
Valery M Dembitsky
DOI10.21767/2471-8521.100005
Valery M Dembitsky*
Department of Chemistry, 901 12th Avenue, Seattle University, Seattle, WA 98122, USA
- *Corresponding Author:
- Valery M Dembitsky
Institute for Drug Discovery
8 Ha-Marpe Str., P.O. Box 45289
Jerusalem 91451, Israel
Tel: +972 526 877 444
E-mail: iddrdo@gmx.com
Received date: August 19, 2015; Accepted date: November 25, 2015; Published date:December 05, 2015
Citation: Dembitsky VM. Bioactive Fungal Endoperoxides. Med Mycol Open Access. 2015, 1:5. doi: 10.21767/2471-8521.100005
Copyright: © 2015 Dembitsky VM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The present review describes research on rare endoperoxides isolated from terrestrial and marine fungi, lichenized fungi and fungal endophytes. More than thirty fungal metabolites have been confirmed to exhibit antimicrobial, antibacterial, and anticancer activities, as well as other activities. A wide spectrum of pharmacological activities is associated with this type of fungal metabolites, which is also true for selected synthetic derivatives.
Keywords
Endoperoxides; Fungi; Fungal endophytes; Lichens; Activities
Introduction
More than 900 endo-peroxides and hydroperoxides have been isolated from natural sources, mainly as constituents of plants, and fungi, fungal endophytes; they also were found in algae, invertebrates, and other organisms [1-5]. Among naturally occurring endo-peroxides and hydroperoxides represented a large group compounds which are shown to possess antimalarial, antibacterial, cytotoxic, and many other activities. In the past several decades, natural peroxides have been isolated from a wide variety of fungi, plants, and marine organisms. Extensive pharmacological screening performed on aquatic and/or terrestrial species resulted in discovery of novel antibacterial, antitumor, and mainly antimalarial agents [6-8]. The purpose of this review is to summarize bioactive metabolites of more than thirty natural endoperoxides, belonging to diverse structural classes: sterols, terpenes, aromatic compounds, and alkaloids.
This paper review new and active endo-peroxides produced by fungi, lichenized fungi, and fungal endophytes described their structures, chemistry, and pharmacological activities.
Sterols and its derivatives
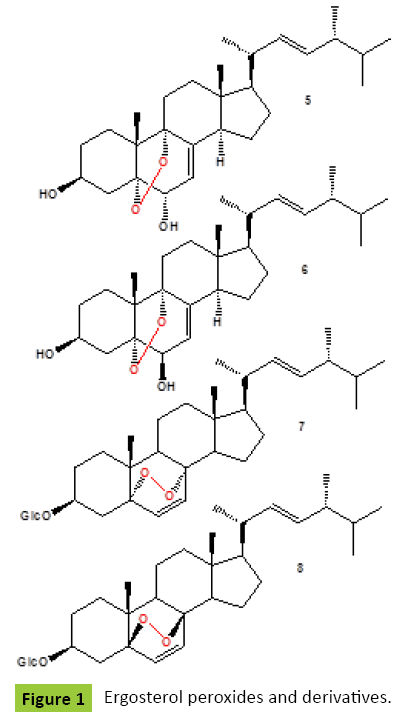
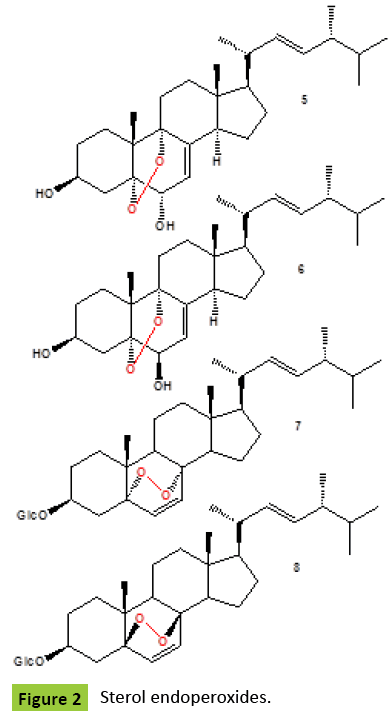
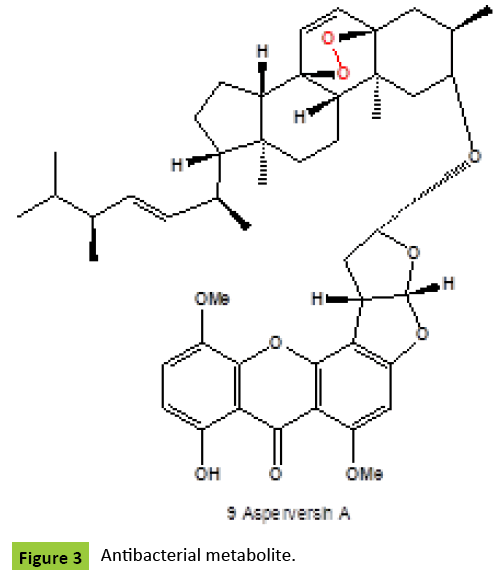
Ergosterol peroxide {1} (Figure 1) has been detected in many fungi and fungal endophytes: Claviceps purpurea [ergot fungus], Ganoderma lucidum, G. tsugae and G. sichuanense [lingzhi mushrooms] [4,5], mushroom Daedalea quercina [4], Piptoporus betulinus [known as the Birch Bracket Mushroom], Cryptoporus volvatus [known as the Grey-Brown Sap Rot Mushroom] [5], Guignardia laricina [Botryosphaeriales], Lampteromyces japonicus [4], a necrotrophic fungus Botrytis cinerea [5], Lactarius uvidus [North American Milk-Cap Mushroom], L. volemus, Cryptoporus volvatus [known as the Grey-Brown Sap Rot Mushroom], Aspergillus sp., A. niger, A. oryzae, A. flavus, A. terreus, and A. fumigatus [5], Fusurium monilforme, F. osysporum, Penicillium rubrum, P. sclerotigenum [4], Dictyonema glabratum [a lichen-forming basidiomycete], Lasiosphaera nipponica, Gloeophyllum odoratum, Gymnopilus spectabilis, Hericium erinaceus [Medicinal, Edible Mushroom], Hypsizigus marmoreus, Inonotus obliquus, I. radiatus, Lenzites betulina [birch mazegill or multicolor gill polypore fungus], fungus Meripilus giganteus, Microporus flabelliformis [syn. Microporus affinis], Naematoloma fasciculare [Yellow Mushroom syn. Hypholoma fasciculare], Phellinus pini [known as Red ring rot or White speck], P. ribis [medicinal fungus], P. torulosus [known as P. torulosus], Roseoformes subflexibilis [wood-rotting fungus], Pyropolyporus fomentarius syn. Fomes fomentarius, Pisolithus tinctorius [known in Europe as the Bohemian truffle], Polyporus tuberaster, and Pseudephebe pubescens [lichenized fungi within the Parmeliaceae family] [5], and from the edible mushroom, Volvariella volvacea [9]. Ergosterol peroxide and its D-glucopyrano-side [2] were obtained from the methanol extract of Cordyceps sinensis [10,11]. The glycosylated form of ergosterol peroxide was found to be a greater inhibitor to the proliferation of K562, Jurkat, WM-1341, HL-60 and RPMI-8226 tumor cell lines [11]. Endophytic fungus No ZZF36 from a brown alga from the South China Sea, produced ergosterol peroxide {1}, along with brassicaterol, and ergosterol [12]. The fruiting bodies of the edible mushroom Gomphus clavatus [family Gomphaceae] were collected from the wild and extracted with solvents of increasing polarity. Crude extracts were evaluated for their total phenolic content, their antioxidant capacity, and their cytotoxic activity against MCF-7 and PC-3 cancer cell lines. Isolated endoperoxide {1} was one of the most active constituents, with IC50 values of 35.8 μM and 30.6 μM for MCF-7 and PC-3 cells, respectively, suggesting that the cytotoxic activity of the crude extract could be at least partly attributed to the presence of ergostan derivatives. Those findings suggest that G. clavatus can be considered as a medicinal food with antioxidant and chemo-preventive activities [13]. Ergosterol peroxide {1} was isolated from the ethanol extract of Pleurotus eryngii, an edible mushroom native to Mediterranean regions of Europe, the Middle East, and North Africa, as an inhibitor of osteoclast differentiation. This compound showed an inhibitory effect in a dose-dependent manner and an inhibition rate of up to 62% with low cytotoxicity, even at a concentration as low as 1.0 μg/ml [14]. Endoperoxide {1} isolated from of the deep-sea derived fungus Aspergillus species CXCTD-06-6a showed activities against P388 and Hela cell lines [15]. Ergosterol peroxide {1} have also been found in some lichenized ascomycetes: Cetraria chlorophylla, C. islandica, Cladonia gracilis, Leioderma pycnophorum, Pseudocyphellaria encoensis and P. pluvialis, Lepolichen coccophorus, Lobaria pulmonaria, Ramalina hierrensis, R. tingitana, Rhizoplaca melanophthalma, Stereocaulon azoreum, Peltigera aphthosa and P. dolichorrhiza [4,5]. Methanol extract of fungus Tremella fuciformis showed significant neuritogenic activity against PC12 cells. Two neuritogenic compounds ergosterol peroxide {1} and 5α,8α-epidioxy-[22E,24R]-ergosta-6,22-diene-3β-ol 3-O-β-D-glucopyranoside {2} were isolated and identified from the methanol extract of fungus T. fuciformis [16]. Same compounds were isolated from the fruiting bodies of the Chinese toxic mushroom Naematoloma fasciculare [17]. Two peroxides {3,4} (Figure 1) produced a camphor fungus ntrodia camphorata [known as Niu-Chang in Taiwan] [18]. Compound {5} obtained from the fruit bodies of mushroom Boletus calopus had weak antifungal action on a few pathogens, such as cucumber wilt disease fungus and wheat scab fungus [19]. Two sterols {5,6} (Figure 2) were isolated from the fruiting body of Panellus serotinus also known as Mukitake mushroom [family Mycenaceae]. Compound {6} was also isolated from other edible mushroom Lepista nuda [also known as blewit, syn. Clitocybe nuda] [20]. Ergostane-type endoperoxy glycosides {7,8} were isolated [21] from the ethanol extract of an attractive mushroom Lactarius volemus [family Russullaceae] which inhibits the growth of several tumor cell lines in vitro [22]. Asperversin A {9} (Figure 3) and ergosterol peroxide {1} was obtained from the culture of Aspergillus versicolor, an endophytic fungus isolated from the marine brown alga Sargassum thunbergii. Compound exhibited antibacterial activities against Escherichia coli and Staphyloccocus aureus [23].
Terpenes and derivatives
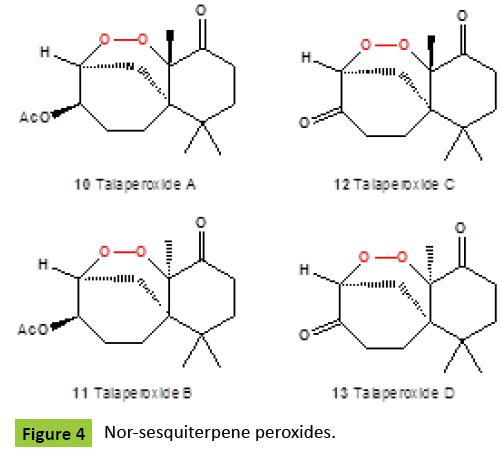
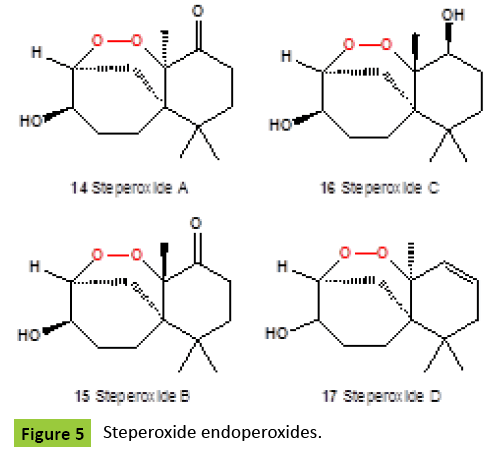
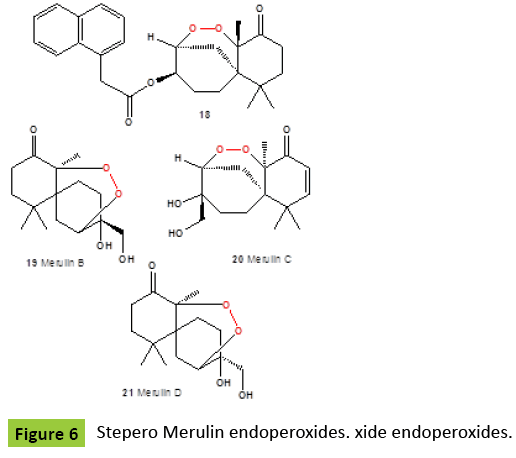
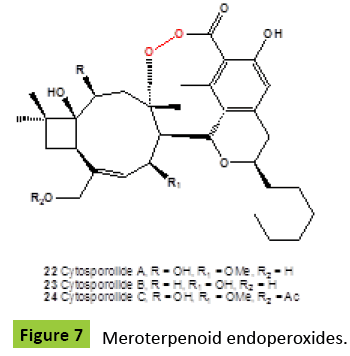
Several nor-sesquiterpene peroxides (Figure 4), named as talaperoxide A {10}, B {11}, C {12}, and D {13}, were isolated from culture of fungi Talaromyces species HN21-3C [family Trichocomaceae]. Isolated compounds showed antineoplastic activity against mammary cancer, prostatic carcinoma, uterine cervix carcinoma or hepatic carcinoma [24,25]. Same compounds, talaperoxides A-D {10-13}, as well as one known analog, steperoxide B {14, or merulin A}, have been isolated from a mangrove endophytic fungus, Talaromyces flavus [26]. Talaperoxides {12} and {13} showed cytotoxicity against the five human cancer cell lines with IC50 values between 0.70 μg/ml and 2.78 μg/ml. Chamigrane-type metabolites named steperoxides A {14}, B {15}, C {16} and D {17}, have been isolated from the hydnoid fungus Steccherinum ochraceum [Phanerochaetaceae]. Compounds {15,17} showed significant antimicrobial activity against Staphylococcus aureus at 10 μg/disk and 5 μg/disk [27- 29]. Semi-synthetic derivative {18} (Figure 5) of the fugallyderived natural product {12} showed the antiparasitic and cytotoxic activity [IC50=0.043 μM vs. T. brucei; IC50=13 μM vs. Hela cells], respectively [30]. Previously, the same nor-chamigrane endoperoxide, named as merulin A [syn. steperoxide B, {15}, B {19}, C {20}, and D {21} (Figure 6), were isolated from the culture broth extract of an endophytic fungus of Xylocarpus granatum. Same compounds were isolated from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala [26]. Metabolites merulin A and C, displayed significant cytotoxicity against human breast [BT474] and colon [SW620] cancer cell lines. Endophytic fungi have been the source of a wide range of structurally interesting and biologically active compounds. The endophytic fungus XG8D, which was isolated from the mangrove plant, Xylocarpus granatum [Meliaceae], as the EtOAc extract of this strain showed potent cytotoxic activity against human breast [BT474] and colon [SW620] cancer cell lines. The fungus strain XG8D was classified as a member of the family Meruliaceae [order Polyporales, subclass Incertae sedis, class Agaricomycetes, phylum Basidiomycota] from rDNA sequences and LUS phylogeny [31]. Cytosporolides A {22}, B {23}, and C {24} (Figure 7), caryophyllene-derived meroterpenoids with a unique peroxylactone skeleton, were isolated from cultures of the fungus Cytospora species. All cytosporolides showed significant antimicrobial activity against the Gram-positive bacteria Staphylococcus aureus and Streptococcus pneumoniae {24}. Also, compound {24} showed good activity in vitro against dermatophytic fungi and moderate activity against C. albicans and S. aureus.
Aromatic endoperoxides
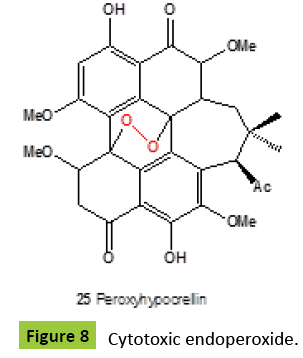
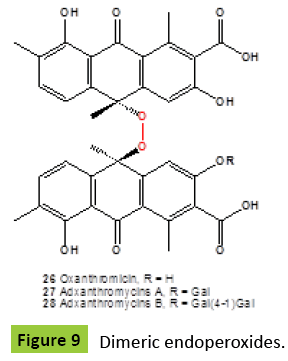
Hypocrellins are dark red pigments having the perylenequinone structure, with photodynamic activity toward microorganisms. These pigments produced by the fungus Hypocrella bambusae [32,33], and a parasitic fungus Shiraia bambusicoia [34-36]. All isolated metabolites have shown anticancer activities [34,37,38], and antiviral activity against the human immunodeficiency virus [HIV-1] [39]. Natural cytotoxic peroxyhypocrellin {25} (Figure 8) was isolated from S. bambusicoia [40]. Rare dimeric anthrone peroxide, named oxanthromicin {23} (Figure 9), an antibiotic isolated from an Actinomadura species SCC 1646 fermentation broth [41,42]. Adxanthromycins A {24} and B {25} were new inhibitors of ICAM-1/LFA-1 mediated cell adhesion molecule isolated from the fermentation broth of Streptomyces species NA-148. Adxanthromycins A and B inhibited the formation of the JY cell aggregates from 1.5 mg/ml, respectively, in a dose dependent manner. Components A and B also inhibited a human T cell leukemia cell line SKW-3 adhesion to soluble ICAM-1 in a dose-dependent manner with an IC50 of 18.8 μg/ml and 25.0 μg/ ml, respectively [43,44].
Spiroketal endoperoxides
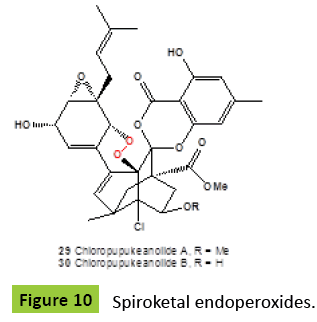
Chloropupukeanolides A {29} (Figure 10) and B {30}, unprecedented spiroketal peroxides, two highly functionalized metabolites featuring a chlorinated pupukeanane core, were isolated from an endophytic fungus Pestalotiopsis fici. The compound {29} was reported to have anti-HIV and anti-cancer activities with an inhibitory effect on HIV-1 replication in C8166 cells of 6.9 μM [EC50 value] and IC50 values of 16.9, 15.5, and 15.9 μM against Hela, MCF-7, and MDA-MB-231 cell lines, respectively [45].
Alkaloid endoperoxides
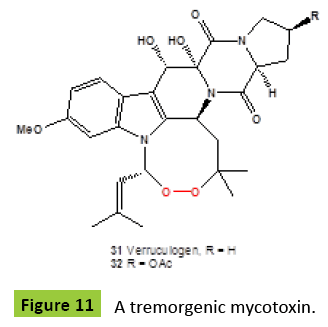
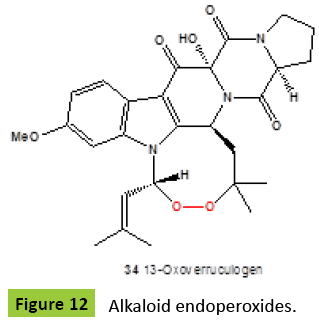
Bioactive endoperoxide verruculogen {31} (Figure 11) for the first time was isolated from a strain of Penicillium verruculosum isolated from peanuts [46]. This compound {31} had also been isolated from a number of other microbiological sources including Aspergillus caespitosus [47], A. fumigatus [48], A. fischeri [49], Penicillium piscarium [50], P. paxilli [51], P. piceum, P. nigricans, P. raistrickii [50], P. estinogenum [52], P. simplicissmum [53], Eupenicillium sp. [54], and an invasive fungal pathogen Neosartorya fischeri [A. fischerianus] [55]. A tremorgenic mycotoxin verruculogen {31} is a potent inhibitor of high conductance Ca activated K [maxi-K] channel, other pharmacological effects of {31} have also been reported [56]. The acetoxy derivative {32} has been isolated from Penicillium verruculosum [57]. More recently, verruculogen {31} and fumitremorgin B were found in several strains of the genus Aspergillus: A. lentulus, A. udagawae, and A. viridinutans [58]. In addition, of eighteen that exerted moderate lethality toward brine shrimps, verruculogen {31} showed significant toxicities with median lethal concentration [LC50] values of 13.6 μg/ml [59]. Other endoperoxide, neurotropic mycotoxin fumitremorgin A {33} (Figure 12), was recovered from a strain of Aspergillus fumigatus [60,61]. Fumitremorgin A {33} known as neurotropic metabolite [62] was produced by fungus Aspergillus fumigatus [63]. Diketopiperazine alkaloid, 13-oxo-verruculogen {34}, had been obtained from the fermentation of Aspergillus fumigatus from a holothurian, Stichopus japonicus [Lingshan Island, Qingdao, China] [64].
Conclusion and Perspectives
During the last 25 years, there has been an unprecedented growth in the chemistry of natural as well as synthetic peroxides [1-5,8,65-71]. Currently, the rapid progress in chemistry of organic peroxides is to a large degree determined by their high biological activity. In medicinal chemistry of peroxides, particular emphasis is given to the design of compounds having activity against causative agents of malaria and human helminthic infections. In medicinal chemistry of peroxides, for example, ascaridole and qinghaosu [known as artemisinin] a natural peroxides exhibiting high antimalarial activity [65,66,70], is the most important drug in use for approximately 25 years. This review also emphasizes the role of endoperoxides from fungi, lichens, and fungal endophytes as an important source of leads for drug discovery.
References
- Dembitsky VM (2008) Bioactive peroxides as potential therapeutic agents.Eur J Med Chem 43: 223-251.
- Dembitsky VM, Gloriozova TA, Poroikov VV (2007) Natural peroxy anticancer agents.Mini Rev Med Chem 7: 571-589.
- Liu DZ, Liu JK (2013) Peroxy natural products. Nat Prod Bioprospect 3: 161-206.
- Casteel DA (1992) Peroxy natural products.Nat Prod Rep 9: 289-312.
- Casteel DA (1999) Peroxy natural products. Nat Prod Rep 16: 55-73.
- Lange BM, Ahkami A (2013) Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes--current status and future opportunities.Plant Biotechnol J 11: 169-196.
- Liu QM, Jiang JG (2012) Antioxidative activities of medicinal plants from TCM. Mini Rev Med Chem 12: 1154-1172.
- Terent'ev AO, Borisov DA, Vil' VA, Dembitsky VM (2014) Synthesis of five- and six-membered cyclic organic peroxides: Key transformations into peroxide ring-retaining products.Beilstein J Org Chem 10: 34-114.
- Mallavadhani UU, Sudhakar AVS, Satyanarayana KVS, Anita Mahapatraa, Wenkui Li, et al. (2006) Chemical and analytical screening of some edible mushrooms. Food Chem 95: 58-64.
- Chao JF, Yin ZQ, Ye WC, Zhao SX (2006) [Chemical constituents from branch of Broussonetiapapyrifera].ZhongguoZhong Yao ZaZhi 31: 1078-1080.
- Bok JW, Lermer L, Chilton J, Klingeman HG, Towers GH (1999) Antitumor sterols from the mycelia of Cordycepssinensis.Phytochemistry 51: 891-898.
- Yang RY, Li CY, Lin YC, Peng GT, Zhou SN (2006) [Study on the sterols from a brown alga endophytic fungus (NO. ZZF36) from the South China Sea].Zhong Yao Cai 29: 908-909.
- Makropoulou M, Aligiannis N, Gonou-Zagou Z, Pratsinis H, Skaltsounis AL, et al. (2012) Antioxidant and cytotoxic activity of the wild edible mushroom Gomphusclavatus.J Med Food 15: 216-221.
- Yokoyama S, Bang TH, Shimizu K, Kondo R (2012) Osteoclastogenesis inhibitory effect of ergosterol peroxide isolated from Pleurotuseryngii.Nat Prod Commun 7: 1163-1164.
- Chang-zhu J, Shi-wei S, Sheng-xin C, Tian-jiao Z, Qian-qun G, et al. (2011) Studies on the active secondary metabolites from the deep-sea-derived fungus Aspergillus sp. CXCTD-06-6a. ZhongguoHaiyangYaowu 30: 1-6.
- Zhang Y, Pei L, Gao L, Huang Q, Qi J (2011) [A neuritogenic compound from Tremellafuciformis].ZhongguoZhong Yao ZaZhi 36: 2358-2360.
- Shi XW, Li XJ, Gao JM, Zhang XC (2011) Fasciculols H and I, two lanostane derivatives from Chinese mushroom Naematolomafasciculare.ChemBiodivers 8: 1864-1870.
- Huang HC1, Liaw CC, Yang HL, Hseu YC, Kuo HT, et al. (2012) Lanostanetriterpenoids and sterols from Antrodiacamphorata.Phytochemistry 84: 177-183.
- Zuo W, Luo DQ (2010) Research on the chemical components of the fruit bodies of Boletus calopus. Anhui NongyeKexue 38: 2356-2357.
- Yaoita Y, Matsuki K, Iijima T, Nakano S, Kakuda R, et al. (2001) New sterols and triterpenoids from four edible mushrooms.Chem Pharm Bull (Tokyo) 49: 589-594.
- Yue JM1, Chen SN, Lin ZW, Sun HD (2001) Sterols from the fungus Lactariumvolemus.Phytochemistry 56: 801-806.
- Zang M, Ying JZ (1994) Economic fungi in the South West of China, Scientific Press. Beijing: 307.
- Miao FP, Li XD, Liu XH, Cichewicz RH, Ji NY (2012) Secondary metabolites from an algicolousAspergillusversicolor strain.Mar Drugs 10: 131-139.
- Li Y, Niu S, Sun B, Liu S, Liu X, et al. (2010) Cytosporolides A-C, antimicrobial meroterpenoids with a unique peroxylactone skeleton from Cytospora sp.Org Lett 12: 3144-3147.
- She Z, Li H, Li M, Zhu X, Lin Y, et al. (2011) Norsesquiterpenoid peroxide with antitumor activity and preparation and application thereof. Chinese Patent: CN 2011-10031487, Faming ZhuanliShenqing.
- Li H, Huang H, Shao C, Huang H, Jiang J, et al. (2011) Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromycesflavus isolated from the mangrove plant Sonneratiaapetala. J Nat Prod 74: 1230-1235.
- Liu DZ, Luo MH (2010) Two new chamigrane metabolites from fermentation broth of Steccherinumochraceum.Fitoterapia 81: 1205-1207.
- Liu DZ, Dong ZJ, Wang F, Liu JK (2010) Two novel norsesquiterpene peroxides from basidiomyceteSteccherinumochraceum. Tetrahedron Lett 51: 3152-3153.
- Li LB,Ren J, Lai R, Cheng ZM, Zhu HJ (2011) Natural cyclic peroxide echinobithiophene A with anti-microbial activity from Echinopsritro L. Chemical Journal of Chinese Universities 32: 891-896.
- Linington R, Navarro G, Pudhom K, McKerrow J (2013) Novel semi-synthetic small molecules for the treatment parasitic disease. Patent: WO 2012-US48743, PCT Int. Appl
- Chokpaiboon S, Sommit D, Teerawatananond T, Muangsin N, Bunyapaiboonsri T, et al. (2010) Cytotoxic nor-chamigrane and chamigraneendoperoxides from a basidiomycetous fungus.J Nat Prod 73: 1005-1007.
- Chen WS, Chen YT, Wan XY, Friedrichs E, et a l. (1981) Structure of hypocrellin and its photooxidation product peroxyhypocrellin. Liebigs Ann Chem 10: 1880-1885.
- Zhenjun D, Lown JW (1990) Hypocrellins and their use in photosensitization.PhotochemPhotobiol 52: 609-616.
- Fang LZ, Qing C, Shao HJ, Yang YD, Dong ZJ, et al. (2006) Hypocrellin D, a cytotoxic fungal pigment from fruiting bodies of the ascomyceteShiraiabambusicola.J Antibiot (Tokyo) 59: 351-354.
- Shen YX, Rong XG, Gao ZH (2002) [Studies on the chemical constituents of Shiraiabambusicola].ZhongguoZhong Yao ZaZhi 27: 674-676.
- Kishi T, Tahara S, Taniguchi N, Tsuda M, Tanaka C, et al. (1991) New perylenequinones from Shiraiabambusicola.Planta Med 57: 376-379.
- Diwu Z (1995) Novel therapeutic and diagnostic applications of hypocrellins and hypericins.PhotochemPhotobiol 61: 529-539.
- Diwu Z, Zhang C, Lown JW (1993) Photosensitization with anticancer agents. 18. Perylenequinonoid pigments as potential photodynamic therapeutic agents: preparation and photodynamic properties of amino-substituted hypocrellin derivatives.Anticancer Drug Des 8: 129-143.
- Hudson JB, Zhou J, Chen J, Harris L, Yip L, et al. (1994) Hypocrellin, from Hypocrellabambuase, is phototoxic to human immunodeficiency virus.PhotochemPhotobiol 60: 253-255.
- Shen YX, Rong XG, Gao ZH (2002) [Studies on the chemical constituents of Shiraiabambusicola].ZhongguoZhong Yao ZaZhi 27: 674-676.
- Patel M, Horan AC, Gullo VP, Loebenberg D, Marquez JA, et al. (1984) Oxanthromicin, a novel antibiotic from Actinomadura. J Antibiot (Tokyo) 37: 413-415.
- Wright JJK, Merill Y, Puar M, McPhail AT (1984) Structure of oxanthromicin (antibiotic 16–550), a novel dimericanthrone peroxide. J ChemSoc, ChemCommun: 473-474.
- Takahashi S, Nakano T, Koiwa T, Noshita T, Funayama S, et al. (2000) Adxanthromycins A and B, new inhibitors of ICAM-1/LFA-1 mediated cell adhesion molecule from Streptomyces sp. NA-148. II. Physico-chemical properties and structure elucidation. J Antibiot (Tokyo) 53: 163-170.
- Nakano T, Koiwa T, Takahashi S, Nakagawa A (2000) Adxanthromycins A and B, new inhibitors of ICAM-1/LFA-1 mediated cell adhesion molecule from Streptomyces sp. NA-148. I. Taxonomy, production, isolation and biological activities. J Antibiot (Tokyo) 53: 12-18.
- Liu L, Niu S, Lu X, Chen X, Zhang H, et al. (2010) Unique metabolites of Pestalotiopsisfici suggest a biosynthetic hypothesis involving a Diels-Alder reaction and then mechanistic diversification.ChemCommun (Camb) 46: 460-462.
- Cole RJ, Kirksey JW, Moore JH, Blankenship BR, Diener UL, et al. (1972) Tremorgenic toxin from Penicilliumveruculosum.ApplMicrobiol 24: 248-250.
- Schroeder HW, Cole RJ, Hein H Jr, Kirksey JW (1975) Tremorgenicmycotoxins from Aspergilluscaespitosus. ApplMicrobiol 29: 857-858.
- Dorner JW, Cole RJ, Hill RA (1984) Tremorgenicmycotoxins produced by Aspergillusfumigatus and Penicillium crustosum isolated from molded corn implicated in a natural intoxication of cattle. J Agric Food Chem 32: 411-413.
- Patterson DS, Shreeve BJ, Roberts BA, MacDonald SM (1981) Verruculogen Produced by soil fungi in England and Wales. Appl Environ Microbiol 42: 916-917.
- Gallagher RT, Latch GC (1977) Production of the TremorgenicMycotoxinsVerruculogen and Fumitremorgin B by Penicillium piscarium Westling.Appl Environ Microbiol 33: 730-731.
- Cockrum PA, Culvenor CCJ, Edgar JA, Payne AL (1979) Chemically different tremorgenicmycotoxins in isolates of Penicillium paxilli from Australia and North America. J Nat Prod 42: 534-536
- Day JB, Mantle PG, Shaw BI (1980) Production of verruculogen by Penicilliumestinogenum in stirred fermenters.J Gen Microbiol 117: 405-410.
- Day JB, Mantle PG (1982) Biosynthesis of radiolabeled verruculogen by Penicillium simplicissimum. Appl Environ Microbiol 43: 514-516.
- Horie Y, Yamazaki M (1985) Production of carcinogenic mycotoxins, sterigmatocystin and its allied compounds, by Emericella species. Trans MycolSoc Japan 26: 411-419.
- Nielsen PV, Beuchat LR, Frisvad JC (1988) Growth of and fumitremorgin production by Neosartoryafischeri as affected by temperature, light, and water activity.Appl Environ Microbiol 54: 1504-1510.
- Nishiyama M, Kuga T (1986) Pharmacological effects of the tremorgenic mycotoxin fumitremorgin A. Jpn J Pharmacol 40: 481-489.
- Uramoto M, Tanabe M, Hirotsu K, Clardy J (1982) A new tremorgenic metabolite related to verruculogen from Penicilliumverruculosum. Heterocycles 17: 349-358.
- Tamiya H, Ochiai E, Kikuchi K, Yahiro M, Toyotome T, et al. (2015) Secondary metabolite profiles and antifungal drug susceptibility of Aspergillusfumigatus and closely related species, Aspergilluslentulus, Aspergillusudagawae, and Aspergillusviridinutans. J Infect Chemother 21: 385-391.
- Li XJ, Zhang Q, Zhang AL, Gao JM (2012) Metabolites from Aspergillusfumigatus, an endophytic fungus associated with Meliaazedarach, and their antifungal, antifeedant, and toxic activities. J Agric Food Chem 60: 3424-3431.
- Yamazaki M, Fujimoto H, Kawasaki T (1980) Chemistry of tremorogenic metabolites. I. Fumitremorgin A from Aspergillusfumigatus.Chem Pharm Bull (Tokyo) 28: 245-254.
- Yamazaki M, Suzuki S, Miyaki K (1971) Tremorgenic toxins from AspergillusfumigatusFres.Chem Pharm Bull (Tokyo) 19: 1739-1740.
- Sanfilippo C, Patti A, Nicolosi G (1999) Enzymatic resolution of (±)-conduritol-B, a key intermediate for the synthesis of glycosidase inhibitors. Tetrahedron: Asymm 10: 3273-3276.
- Dembitsky VM (2003) Oxidation, epoxidation and sulfoxidation reactions catalysed by haloperoxidases. Tetrahedron 59: 4701-4720.
- Wang CF (2008) Studies on the chemical constituents about three plants of Compositae and one plant of Thymelaeaceae. PhD thesis. Zhejiang University, China
- Dembitsky VM (2015) Natural hydroperoxides as potential terapeutical agents. SDRP J Plant Sci 1: 1-9.
- Dembitsky VM (2015) Astonishing diversity of natural peroxides as potential therapeutic agents. J Mol Genet Med 9: 1-18.
- Dembitsky VM (2004) Chemistry and biodiversity of the biologically active natural glycosides. ChemBiodivers 1: 673-781.
- Dembitsky VM, Shkrob I, Dor I (1999) Separation and identification of hydrocarbons and other volatile compounds from cultured blue-green alga Nostoc sp. by gas chromatography–mass spectrometry using serially coupled capillary columns with consecutive nonpolar and semipolar stationary phases. J Chromatog A 862: 221-229.
- Vizer SA, Sycheva ES, Al Quntar AA, Kurmankulov NB, Yerzhanov KB, et al. (2015) Propargylic sulfides: synthesis, properties, and application. Chem Rev 115: 1475-1502.
- Dembitsky V, Shkrob I, Hanus LO (2008) Ascaridole and related peroxides from the genus Chenopodium.Biomed Pap Med FacUnivPalacky Olomouc Czech Repub 152: 209-215.
- Terent'ev AO, Platonov MM, Levitsky DO, Dembitsky VM (2011) Organosilicon and organogermanium peroxides: synthesis and reactions. Russian Chem Rev 80: 807-828.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences