Candida blankii Candidaemia: A Case Report of an Emergent Opportunistic Pathogen with Reduced Susceptibility.
1Department of Primary Health, Cliniques Universitaires Saint-Luc, Avenue Hippocrate Brussels, Belgium
2CHR Mons-Hainaut, Site Warquignies–rue des Chaufours Boussu, Belgium
*Corresponding Author:
- Ekaterina MelnikDepartment of Primary Health, Cliniques Universitaires Saint-Luc, Avenue Hippocrate Brussels, Belgium E-mail:ekaterina.melnik@jolimont.be
Received date: May 02, 2022, Manuscript No. IPMMO-22-13379; Editor Assigned date: May 04, 2022, PreQC No. IPMMO-22-13379(PQ); Reviewed date: May 16, 2022, QC No. IPMMO-22-13379; Revised date: May 26, 2022, Manuscript No. IPMMO-22-13379(R); Published date: June 01, 2022, DOI: 10.36648/2471-8521.8.3.028
Citation: Melnik E (2022) Candida blankii Candidaemia: A Case Report of an Emergent Opportunistic Pathogen with Reduced Susceptibility. Med Mycol Open Access Vol.8 No.3:28
Abstract
Due to the growing number of critically ill patients, candidaemia became one of the most frequent causes of invasive fungal infections. It causes an overall mortality of more than 50% in hospitalized patients and is associated with prolonged hospital stay and high healthcare costs. Five species of Candida (C. albicans, C. glabrata, C. tropicalis, C. krusei and C. parapsilosi) account for more than 90% of all diagnosed cases, but less frequent species causing candidaemia are described.
Our report highlights the emergence of C. blankii, an opportunistic pathogen with reduced susceptibility to Fluconazole and Echinocandins. The therapeutic experience is limited but it is more prudent to avoid these agents for treatment of C. blankii infections. Posaconazole, Voriconazole and Amphotericin B seem to be the first line therapy to use.
Keywords:C. albicans; C. glabrata; C. tropicalis; C. parapsilosi; Posaconazole
Introduction
Candida spp. is the main cause of invasive fungal infections in hospitalized patients [1]. Widespread increase in these infections is predominately due to medical progress (increased prevalence of susceptible hosts receiving corticotherapy, immunosuppressive therapy for organ transplantation, broad-spectrum antibiotics and use of prophylactic or empiric antifungal therapy). The most frequent of invasive candidiasis is candidaemia, a clinical condition with an overall mortality of more than 50%. The prognosis for candidaemia is comparable to that of septic shock, but in recent years the proportion of non-C. albicans strains resistant to triazole has significantly increased [2].
In 2015, Zaragoza et al. reported a 14-year-old teenage boy with cystic fibrosis presenting repeated pulmonary exacerbations with Candida blankii [3]. Since, two other reports (a 27-week-old preterm neonate with necrotizing enterocolitis and a 16-year-old female with bilateral lung transplantation for cystic fibrosis) have hignlighted the clinical relevance of C. blankii in human infections [4,5]. Here we described another case of C. blankii candidaemia diagnosed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry on yeast colonies from blood cultures and confirmed by sequence analysis of the internal transcribed spacer.
Case Description
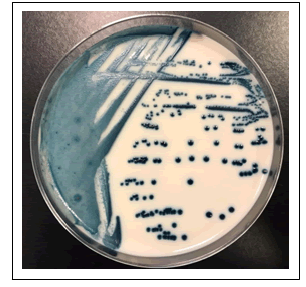
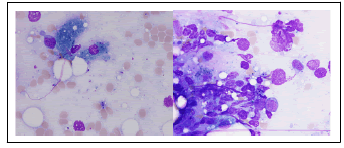
The patient was a 34-year-old male hospitalized for fever of unknown origin. His medical history was notable for X-linked chronic granulomatous disease (a hereditary disorder of host defense due to absent or decreased activity of phagocyte NADPH oxidase), ulcerative colitis, Rendu-Osler-Weber disease and multiple episodes of pulmonary aspergillosis under antifungal prophylaxis consisting of itraconazole 100 mg per day. Blood cultures were collected (Bactec plus Aerobic/F and Bactec Lytic/10 Anaerobic/F, Becton Dickinson) and became positive for yeasts after 30 hours of incubation (aerobic bottle). Fluconazole was prescribed due to the report of yeasts on blood cultures. The yeast isolate initially presented pink colonies wich turned into dark blue (similar to Candida tropicalis) on CHROMagar Candida after 24 hours incubation (Figure 1) and were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI Biotyper, Bruker Daltonics) as Candida blankii CBS 1898T (score value: 1,97). The yeast identification was confirmed by sequence analysis of the internal transcribed spacer (ITS 1 and ITS 2, MycoBank Database). The antigungal treatment was switched to Anidulafungine due to the yeast identification. Antifungal susceptibility testing was carried out using broth microdilution method (Sensititre YeastOne YO10, thermo scientific) and the minimal inhibitory concentration values were: Anidulafungin=0.25 µg/mL, Micafungin=0.12 µg/mL, Caspofungin=0.25 µg/mL, 5-Flucytosine=0.06 µg/mL, Posaconazole=1 µg/mL, Voriconazole=0.5 µg/mL, Itraconazole=0.5 µg/mL, Fluconazole=16 µg/mL and Amphotericin B=1 µg/mL. (Figure 2). Because the isolate showed reduced susceptibility to Caspofungine, Anidulafungine and Fluconazole, the treatement was shifted to Voriconazole and Amphotericin B. Afer 4 days antifungal therapy, blood cultures were still positive and the patient was transferred to intensive care unit. A bone marrow aspiration was realised for pancytopenia and showed yeast invasion (Figure 3). Despite the treatment, the patient developed a multiple organ failure and died on day 7 of hospitalization.
Results and Discussion
Candidaemia is one of the most frequent causes of bloodstream infections and is associated with high morbidity and mortality, prolonged hospital stay and high healthcare costs [6-8]. Blood cultures (current diagnostic gold standard) are positive for Candida spp. in less than 50% of candidaemia [9] and explain the poor patient outcomes. Moreover, candidaemia incidence is increasing because of the growing number of critically ill patients [2,10]. Five species of Candida (C. albicans, C. glabrata, C. tropicalis, C. krusei and C. parapsilosi) account for more than 90% of all diagnosed cases [11], but less frequent species causing candidaemia are described.
Our report highlights the emergence of C. blankii, an opportunistic pathogen with reduced susceptibility to Fluconazole and Echinocandins. The therapeutic experience is limited (one other case of bloodstream infection and one case of pulmonary exacerbation) but the two other case reports show the same data concerning the minimal inhibitory concentrations.
Conclusion
In conclusion, it is more prudent to avoid Fluconazole and Echinocandins for the treatment of C. blankii infections. Posaconazole, Voriconazole and Amphotericin B seem to be the first line therapy to use.
Refernces
- Magalhaes Y, Bomfim MR, Melonio L, Riberio P, Cosme L, et al. (2015) Clinical significance of the isolation of Candida species from hospitalized patients. Braz J Microbiol 46: 117–123.
[Crossref], [Google Scholar], [Indexed]
- Eggimann P, Pittet D (2002) Candidoses en réanimation. Réanimation 11: 209-21.
[Crossref], [Google Scholar]
- Zaragota S, Galanternik L, Vazquez M, Teper A, Cordoba S, et al. (2015) 318 Candida blankii: New agent in cystic fibrosis airways? J Cyst Fibros 14: 140.
[Crossref], [Google Scholar]
- Nobrega de Almeida J, Campos S, Thomaz D, Thomaz L, de Almeida R, et al. (2018) Candida blankii: An emergent opportunistic yeast with reduced susceptibility to antifungals. Emerg Microbes Infect 7: 24.
[Crossref], [Google Scholar], [Indexed]
- Al-Haqqan A, Al-Sweih N, Ahmad S, Khan S, Joseph L, et al. (2018) Azole-resistant Candida blankii as a newly recognized cause of bloodstream infection. New Microbes New Infect 26: 25-29.
[Crossref], [Google Scholar], [Indexed]
- Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, et al. (2004) Epidemiology of candidemia in swiss tertiary care hospitals: Secular trends 1991-2000. Clin Infect Dis 38: 311-320.
[Crossref], [Google Scholar], [Indexed]
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, et al. (2003) Attributable mortality of nosocomial candidemia, revisited. Clin Infecti Dis 37: 1172-1177.
[Crossref], [Google Scholar], [Indexed]
- Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, et al. (2005) Excess mortality, hospital stay, and cost due to candidemia: A case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol 26: 540-547.
[Crossref], [Google Scholar], [Indexed]
- Clancy CJ, Nguyen MH (2013) Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infecti Dise 56: 1284-1292.
[Crossref], [Google Scholar], [Indexed]
- Bitar D, Lortholary O, Le Strat Y, Nicolau J, Coignard B, et al. (2014) Population-based analysis of invasive fungal infections, France, 2001-2010. Emerg Infect Dis 20: 1149-1155.
[Crossref], [Google Scholar], [Indexed]
- Guinea J (2014) Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20: 5-10.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences