Cryptococcosis in Dogs: A Case Report in a Labrador retriever in Bogota, Colombia
Felipe Perez, Oscar Avila, Norida Velez and Patricia Escandon
DOI10.21767/2471-8521.100004
Felipe Pérez1, Oscar Avila1, Nórida Vélez2 and Patricia Escandón2*
1Clínica Veterinaria Dover, Calle 126A # 7-98, Bogotá 110111, Colombia
2Grupo de Microbiología, Instituto Nacional de Salud, Calle 26 # 51-20, Bogotá 110111, Colombia
- *Corresponding Author:
- Patricia Escandón
Grupo de Microbiología, Instituto National de Salud
Calle 26 # 51-20, Bogotá 110111, Colombia
Tel: +571 2207700
Fax: +571 2207700
E-mail: pescandon@ins.gov.co
Received date: September 28, 2015; Accepted date: November 13, 2015; Published date: November 26, 2015
Citation: Escandon P, et al. Cryptococcosis in Dogs: A Case Report in a Labrador retriever in Bogotá, Colombia. Med Mycol Open Access. 2015, 1:4. doi: 10.21767/2471-8521.100004
Copyright: © 2015 Escandon P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cryptococcosis naturally occurs in animals, and becomes a life-threatening disease in species such as cats, dogs, horses and koalas. This is the first case of cryptococcosis in a three-year-old Labrador retriever dog reported in Colombia, with description of the clinical case, characterization of the fungal strain and search of the fungus in the environment surrounding the animal. Cryptococcus neoformans VNI was isolated from animal samples, as well as from Eucalyptus trees present in the environment surrounding the dog.
Keywords
Cryptococcosis; Dogs; Var grubii; Environment
Introduction
Cryptococcosis is now being considered an important infectious disease both in humans and animals, caused by two species of yeasts of the genus Cryptococcus: Cryptococcus neoformans and C. gattii, isolated from environmental sources such as plant material and avian excreta, where the fungus finds its ecological niche to reproduce, disperse and become the possible source of infections for the host [1]. Disease may occur naturally in a wide range of animal species (birds, marsupials, ferrets), which may become more susceptible to acquiring the disease, as the vast majority of these animals reside close to environmental sources that may harbour the fungus, being exposed to this pathogen [2]. As in cats, dogs could be affected by acute and chronic upper respiratory infections that may facilitate primary colonization and/or invasion of the nasal cavity by cells of C. neoformans/C. gattii, disrupting natural barriers of the animals, thus allowing colonization by the fungus. Some studies have demonstrated that involvement of the central nervous system (CNS) and disseminated disease is more common to find in different breeds [3]. Definitive diagnosis of the disease is made when the fungus is observed by cytological and/or histological examination, or culture of C. neoformans/C. gattii from a normally sterile site, being the latter a more sensitive test [4]. We report a case of cryptococcosis in a Labrador retriever male dog, with characterization of the fungal strain and environmental exposure of the animal.
Cryptococcosis is now being considered an important infectious disease both in humans and animals, caused by two species of yeasts of the genus Cryptococcus: Cryptococcus neoformans and C. gattii, isolated from environmental sources such as plant material and avian excreta, where the fungus finds its ecological niche to reproduce, disperse and become the possible source of infections for the host [1]. Disease may occur naturally in a wide range of animal species (birds, marsupials, ferrets), which may become more susceptible to acquiring the disease, as the vast majority of these animals reside close to environmental sources that may harbour the fungus, being exposed to this pathogen [2]. As in cats, dogs could be affected by acute and chronic upper respiratory infections that may facilitate primary colonization and/or invasion of the nasal cavity by cells of C. neoformans/C. gattii, disrupting natural barriers of the animals, thus allowing colonization by the fungus. Some studies have demonstrated that involvement of the central nervous system (CNS) and disseminated disease is more common to find in different breeds [3]. Definitive diagnosis of the disease is made when the fungus is observed by cytological and/or histological examination, or culture of C. neoformans/C. gattii from a normally sterile site, being the latter a more sensitive test [4]. We report a case of cryptococcosis in a Labrador retriever male dog, with characterization of the fungal strain and environmental exposure of the animal.
Clinical case
Clinical report
Canine male patient, three years of age belonging to the Labrador retriever breed, permanently living in Bogotá, capital city of Colombia with daily visits to a natural park, visiting the countryside in a region with high density of Eucalyptus trees once a week, was presented to veterinary attention with a mass in the left submandibular region. Physical exploration revealed an increase in the size of the mass and local temperature of the affected area; the mass was punctured obtaining a purulent fluid. Abscess drainage was performed with a Penrose drain and primary antibiotic treatment was initiated with Enrofloxacin (5 mg/kg), Meloxicam as an antiinflammatory (0.1 mg/kg) and local cleansing with Chlorhexidine (0.5%) during one week. After several veterinary controls, on day +19 the animal was directed again to the service with the re-appearance of the mass, apathy and lymphadenomegaly of submandibular lymph nodes. A cytological sample was taken revealing a neutrophic inflammatory reaction, providing once again antibiotic treatment with Enrofloxacin and homotoxicological therapy with Traumeel for one week. On day +63 the animal was redirected to the veterinary service showing apathy, subcutaneous tissue inflammation in the submandibular region and cough. The patient was hospitalized and complementary blood exams were performed [complete blood Count (CBC) and blood chemistry screen], with findings of anaemia and mild leucocytosis. Cytological examinations were performed on day +75, reporting fibrosis. Chest radiographs were taken, finding a mild bronchial pattern. Antibiotic treatment was complemented with Metronidazole (7.5 mg/kg) and daily vaporizations with Gentamycin, N-Acetyl Cysteine and dexamethasone. Due to the mild response to treatment and the chronicity of the disease, a third cytological examination was taken on day +86, observing cells compatible with a mycological infection, and antifungal therapy was added with Ketoconazole (5 mg/kg) and Fluconazole (10 mg/ kg). Patient begins with neurological signs on day +90 (Nystagmus, Raving, Seizures), and on day +92, owners decided to euthanize the dog.
Diagnostic procedure
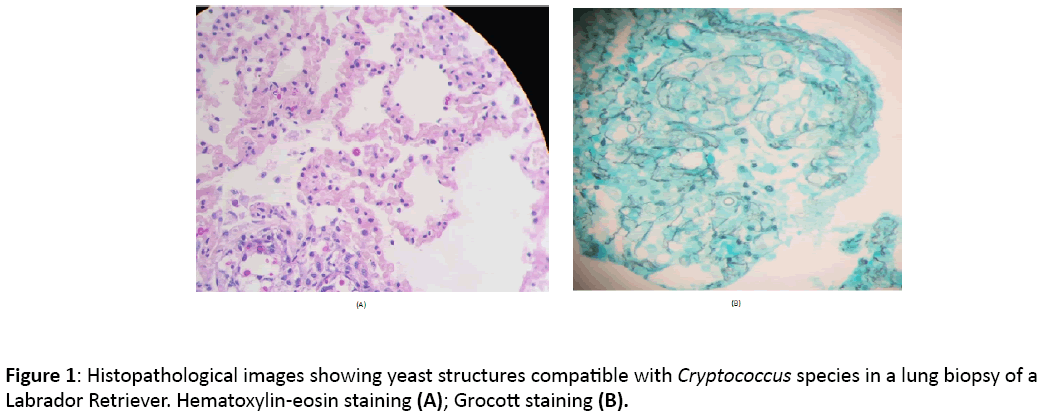
After performing two cytological examinations of the mass in the submandibular region on days +19 (inflammatory reaction) and +75 (fibrosis), where the observed cellularity was compatible with a chronic inflammatory process, CBC revealed anaemia and mild neutrophil leucocytosis (HCT 33.6% and WBC 21.900 μl) and normal blood chemistry, cytological examination was again performed on day +86, since no response to treatment was observed. On the same day a fineneedle aspiration was done in a peri-ocular subcutaneous mass. These cytological examinations revealed cells compatible with the genus Cryptococcus. Once cryptococcosis was included into the differential diagnosis, biopsies were taken for mycological culture and blood samples for detection of the capsular antigen of Cryptococcus to confirm the suspicion of a mycotic infection. Chest RX were also taken, but no finding was compatible with a pulmonary mycosis. An abdominal echography was performed, revealing splenomegaly and diffuse hepatomegaly and mesenteric lymphadenomegaly. Mild leukocytosis persisted (24.000 μl) and Babesia species was diagnosed. At this moment, blood chemistry results were compatible with a cholangiohepatitis (ALT 874 U/L, Alkaline phosphatase 1777 U/L, GGT 33 U/L). Biopsy examination revealed a dermatitis and granulomatous panniculitis of mycotic origin, suggesting as first differential diagnosis Cryptococcus species or Blastomyces species Culture revealed growth of Cryptococcus neoformans var grubii, identified through matrix assisted laser desorption ionization time-of-flight (MALDI-TOF) technique, result that was then confirmed by conventional phenotypic tests and molecular type identification, resulting in the characterization of the molecular pattern VNI by PCR fingerprinting and restriction fragment length polymorphism (RFLP) of the URA5 gene. Cryptococcal capsular antigen titers ≥ 1:1024 were found when serologic testing was done. After euthanasia on day +92, necropsy was performed reporting a systemic mycosis with splenic, lymph nodes, lungs and bone marrow involvement. Grocott and Haematoxylin and Eosin (H&E) staining confirmed the presence of Cryptococcus species (Figure 1). Brain sections were not examined in the necropsy.
Environmental study
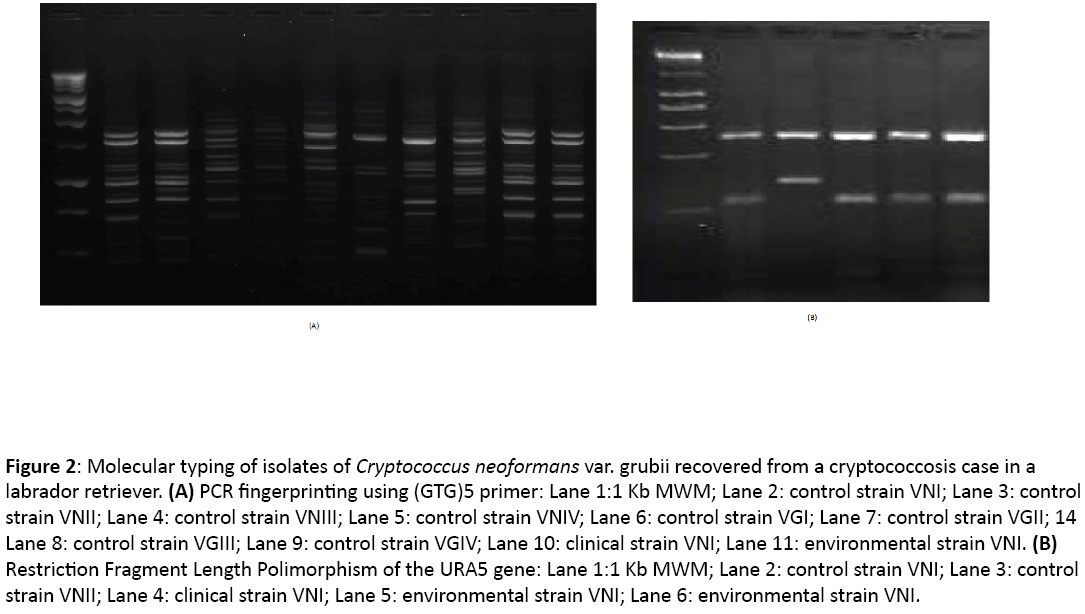
Two hundred and six environmental samples of Eucalyptus species (n=141), Pinus species (n=61) and excreta from Hirundo rustica (n=4) were collected in the surroundings of a house in the countryside where the animal spent at least one day of the week (167 samples were taken in this area), as well as in a natural park in Bogotá where the dog was taken on a daily basis (39 samples collected). Samples from detritus, fruit, leaves and bark were taken from both Eucalyptus and Pinus trees, and bird excreta were processed using conventional techniques [5] ; two environmental isolates were recovered from the cortex and fruit from two Eucalyptus species trees present at the countryside, and were identified as C. neoformans using conventional techniques and were stored as glycerol stocks at -70°C. The clinical isolate and two environmental strains were characterized by molecular techniques to determine the molecular pattern of each isolate by using PCR fingerprinting and restriction fragment length polymorphism (RFLP) of the URA5 gene [6]. The three isolates were typed as C. neoformans var. grubii, molecular type VNI (Figure 2).
Figure 2: Molecular typing of isolates of Cryptococcus neoformans var. grubii recovered from a cryptococcosis case in a labrador retriever. (A) PCR fingerprinting using (GTG)5 primer: Lane 1:1 Kb MWM; Lane 2: control strain VNI; Lane 3: control strain VNII; Lane 4: control strain VNIII; Lane 5: control strain VNIV; Lane 6: control strain VGI; Lane 7: control strain VGII; 14 Lane 8: control strain VGIII; Lane 9: control strain VGIV; Lane 10: clinical strain VNI; Lane 11: environmental strain VNI. (B) Restriction Fragment Length Polimorphism of the URA5 gene: Lane 1:1 Kb MWM; Lane 2: control strain VNI; Lane 3: control strain VNII; Lane 4: clinical strain VNI; Lane 5: environmental strain VNI; Lane 6: environmental strain VNI.
Discussion
Cases of cryptococcosis in dogs are characterized mainly by the presentation of a systemic dissemination of infection, resulting in life threatening illness. The disease may occur in any age, but the majority of the cases have been reported in dogs younger than 6 years of age [7,8], as the case presented here of cryptococcosis in a three year old Labrador retriever dog. A report on breed predisposition to the disease has also been reported, however, the molecular mechanisms that may explain this phenomenon have not been elucidated. Cryptococcosis in dogs manifest with clinical signs, which depend on the sites of infection, but it is frequent to have involvement of the CNS, eyes, gastrointestinal tract and adrenal glands, with over 80% of dogs with disseminated disease having CNS involvement [9,10]. In the United States, dissemination of disease is more prevalent compared to cases in Australia, where nasal involvement without dissemination is more common. Access of the fungus to the CNS in the case presented here may have been through the cribriform plate to the olfactory bulb or via haematogenous route as described in other cases of cryptococcosis in dogs [11], leading to the onset of neurological signs.
According to Castellá et al. [12], a negative result in the cytology does not rule out the possibility of an infection. In the slides that were analysed on days +19 and +75 from the submandibular mass, the yeast was not detected possibly because of the great number of inflammatory cells and a low tissue infiltration of the fungus. It was necessary to perform a third cytology on day +86 in order to be able to detect the fungal cells. Staining with Indian ink was not used, although it is recommended in the diagnosis of cases of Cryptococcosis [13], because there was no suspicion of a fungal infection previous to the third cytology, which was taken due to the diminished response to treatment, and resulted in a definitive test to obtain an accurate diagnosis. Growth of the fungus in culture, identification by histopathology and the presence of antigenic titters above 1:2 in animal serum samples complement the diagnosis of this disease, and in animals with disseminated cryptococcosis, these titers may be very high (1:60,000) [4,14]. The presence of fungal infection was evident from the result of the diagnostics tests performed, except for the chest RX, where no pulmonary pattern matching a mycosis was observed. Culture of the organism from tissue, CSF or body fluids provides a definite diagnosis of the disease; differentiation of the species of Cryptococcus neoformans or gattii, is important since in areas such as British Columbia, Canada, infections in animals due to C. gattii are mandatory to be reported to the public health authorities, as a consequence of the cryptococcosis outbreak reported [15]. For epidemiological purposes, determination of the molecular type is also relevant; in a study performed in the pacific northwest of the United States, dogs with cryptococcosis are infected with C. gattii VGII, the same genotype mostly recovered from the Vancouver Island outbreak [7,8]. In eastern Australia, cryptococcosis in animals is prevalent, reporting that 18% of dogs with cryptococcosis were infected with C. gattii [16]. The recovery of VNI from this dog is consistent with the reports in the United States, with the exception of the Pacific Northwest, in which most dogs with cryptococcosis are infected with the variety grubii VNI, whilst C. gattii is more prevalent as a cause of disease in cats [4]. The outbreak presented on Vancouver Island, British Columbia demonstrated that animals serve as sentinels for the disease in people; as for all molecular cryptococcal molecular types, zoonotic transfer to people from an infected animal is not likely to occur, since in the animal, the organism exists in the yeast form, different from the infectious spores which are thought to be found in the environment, becoming the possible source of infection for humans [17]. Therefore, environmental sampling plays an important role in determining the possible source to which the animal was exposed. In this report, possible exposure to Eucalyptus trees may have been the source from which the animal acquired the disease. In Colombia, the first case of cryptococcosis in a Cocker spaniel dog was reported in 1987, as a cryptococcal nasal granuloma diagnosed by histopathological study, microbiological test, pathogenicity and immunofluorescence test [18]. This is the first report of cryptococcosis in a Labrador retriever dog in Colombia, demonstrating that the disease should also be included as differential diagnosis in animals, not only as a tool to treat animal patients accurately, but also because animals act as sentinels for detecting cryptococcosis in human patients.
Acknowledgement
Dr. Oscar Benavides, veterinary clinician from the Clínica Veterinaria Dover, who attended the case, facilitating the clinical history of the animal. Corpavet Laboratory for the histopathological procedures. Zoodiagnostic Laboratory and Unidad de Investigación en Proteómica y Micosis humanas, Pontificia Universidad Javeriana for culture isolation and identification of the strain.
Conflict of Interest
The author has no conflict of interest.
References
- Levitz SM, Boekhout T (2006) Cryptococcus: The once-sleeping giant is fully awake. FEMS Yeast Res 6: 461-462.
- Malik R, Krockenberger M, O´Brien C, Carter D, Meyer W, et al. (2011) Veterinary insights into cryptococcosis caused by Cryptococcus neoformans and Cryptococcus gattii. In: Cryptococcus from human pathogen to model yeast. Washington DC: ASM Press: 489-504.
- Malik R, Hunt GB, Bellenger CR, Allan GS, Martin P, et al. (1999) Intra-abdominal Cryptococcosis in two dogs. J Small AnimPract 40: 387-391.
- Vorathavorn V, Sykes J, Feldman D (2013) Cryptococcosis as an emerging systemic mycosis in dogs. J Vet EmergCrit Care 23: 489-497.
- Escandón P, Sanchez A, Firacative C, Castañeda E (2010) Isolation of Cryptococcus gattii molecular type VGIII, from Corymbiaficifolia detritus in Colombia. Med Mycol 48: 675-678.
- Escandón P, Sánchez A, Martínez M, Meyer W, Castañeda E (2006) Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res 6: 625-635.
- Lester SJ, Malik R, Bartlett KH, Duncan CG (2011) Cryptococcosis: update and emergence of Cryptococcus gattii. Vet ClinPathol 40: 4-17.
- Lockhart S, Iqbal N, Harris J, Grossman N, DeBess E, et al. (2013) Cryptococcus gattii in the United States: Genotypic diversity of human and veterinary isolates. Plos One 8: e74737.
- Trivedi SR, Sykes JE, Cannon MS, Wisner ER, Meyer W, et al. (2011) Clinical features and epidemiology of cryptococcosis in cats and dogs in California: 93 cases (1988–2010). J Am Vet med Assoc 239: 357-369.
- Berthelin CF, Bailey CS, Kass PH, Legendre AM, Wolf AM (1994) Cryptococcus of the nervous system in dogs, part 1: epidemiologic, clinical and neuropathologic features. Prog Vet Neurol 5: 88-97.
- Sykes JE, Sturges BK, Cannon MS, Gericota B, Higgins RJ, et al. (2010) Clinical signs, imaging features, neuropathology, and outcome in cats and dogs with central nervous system cryptococcosis from California. J Vet Intern Med 24: 1427-1438.
- Castellá G, AbarcaML, Cabañes JF (2008) Criptococosis y animales de compañía. Rev IberoamMicol25: 19-24.
- Tomas TB (2002) Epidemiología de la criptococosis en España. Caracterización de los aislados de Cryptococusneoformans. Universidad Autónoma de Barcelona. Barcelona, España: 1-115.
- Lappin M, Turnwald G (2004) Microbiology and infectious diseases. In: Willard M, Tvedten H (eds.). Small animal clinical diagnosis by laboratory methods. (4thedn.) Missouri, pp. 333-357.
- Stephen C (2002) Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J 43: 792-794.
- O’Brien CR, Krockenberger MB, Wigney DI, Martin P, Malik R (2004) Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med Mycol 42: 449-460.
- Datta K, Bartlett KH, Baer R, Byrnes E, Galanis E (2009) Spread of Cryptococcus gattii into Pacific Northwest region of the United States. EmergInfect Dis 15: 1185-1191.
- Botero F, Ferreira G, Orrego F (1987) Cryptococcal nasal granuloma in a dog. First report in Colombia. ActualidadesBiológicas 16: 125-127.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences