Morpho-Molecular Characterization and Virulence Determination of Entomopathogenic Fungal Isolates Native to Indian Subcontinent
Sapna Mishra, Peeyush Kumar and Anushree Malik
DOI10.21767/2471-8521.100003
Sapna Mishra, Peeyush Kumar and Anushree Malik*
Applied Microbiology Laboratory, Centre for Rural Developmentand Technology, Indian Institute of Technology, Delhi, New Delhi-110 016, India
- *Corresponding Author:
- Anushree Malik
Applied Microbiology Laboratory
Centre for Rural Developmentand Technology, Indian Institute of Technology
Delhi, New Delhi-110 016, India
Tel: +91-11-26591158
Fax: +91-11-26591121
E-mail: anushree_malik@yahoo.com
Received date: August 13, 2015; Accepted date: November 13, 2015; Published date: November 24, 2015
Citation: Malik A, et al. Morpho-Molecular Characterization and Virulence Determination of Entomopathogenic Fungal Isolates Native to Indian Subcontinent. Med Mycol Open Access. 2015, 1:3. doi: 10.21767/2471-8521.100003
Copyright: © 2015 Malik A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The house fly, Musca domestica L. is a major pest of medical and veterinary significance which due to their ubiquitous lifestyle and broad food preference achieves a very high density in favorable climatic conditions of Indian subcontinent. This has led to several instances of severe health condition among human and animal population, calling attention towards M. domestica control through existing and innovative methods. Use of biological control agent, such as entomopathogenic fungi (ENPF) for M. domestica control has shown potential, and need to be further explored for the newer isolates suited to particular environment for greater efficacy. The present study attempted the isolation and identification of native ENPF strains suitable for M. domestica control. Method and findings: Five strains of ENPF were isolated from soil samples, which on preliminary identification of morphological, microscopic and spore attributes were determined to be isolates of Beauveria species. The isolates showed wide variation in pathogenicity against M. domestica, with 72.7%-100% and 36.7%-72.3% mortality against adults and larvae life stage, respectively. Isolate ‘1’ depicting maximum insecticidal activity against M. domestica was selected for molecular identification using 18s-rRNA, and found to be Beauveria bassia HQ917687. The molecular analysis using random amplified polymorphic DNA (RAPD) showed isolate ‘2’ to be most similar to isolate ‘3’, while isolate ‘1’ and ‘5’ showed maximum variation between them. RAPD result was in conformity with various other properties of isolates; such as biomass, spore yield, hydrophobicity and insecticidal activity. Conclusion: The variations in activity obtained through pathogenicity assessment of fungal isolates in this study reflected the strain diversity of Beauveria isolates. The results also signified the importance of strain selection for effective control measures as well as for further consideration of commercial aspects.
Keywords
Entomopathogenic fungi; Isolation; Characterization; Pathogenicity; Musca domestica
Introduction
Entomopathogenic fungi (ENPF) due to their broad host range, diminution of potential environmental hazards and theoretical impossibility of resistance development in target insects, are advantageous for pest management strategies [1]. Also, due to their multiple modes of action for insect kill and ability of adapt to pertaining environmental conditions, ENPFs have emerged as bio pesticide of considerable potential. The literature is replete with studies employing potential of ENPFs (e.g., Entomophthora muscae, Beauveria bassiana and Metarhizium anisopliae etc.) for insect control [2-5]. Most of these studies reports use of ENPFs for control of agricultural pests [2,3]. However, some other studies depict ENPF activity against pests of vector significance, such as mosquitoes and house fly [4,5].
The house fly, Musca domestica L. (Diptera: Muscidae) is a significant vector of more than 100 medical and veterinary pathogens which due to their ubiquitous lifestyle and broad food preference achieves a very high density in favourable climatic conditions of Indian subcontinent, leading to environment and health menace [1]. The vector potential of M. domestica has been proved for the propagation of protozoan (amoebic dysentery), bacterial (shigellosis, salmonellosis, cholera), helminthic (round worms, hookworms, pinworms and tapeworms), viral (Turkey Corona virus, Reticuloendotheliosis virus, H5N1 influenza virus) and rickettsial infections, among others [4]. The study by Barro et al. [6], revealed M. domestica to be predominant fly associated with street-food vendors in Ouagadougou which carried isolates of Coliform, Salmonella, Shigella, Staphylococci and Streptococci on both their legs and proboscis, while Barin et al. [7], demonstrated presence of Newcastle disease virus in the significant number of adult muscid flies collected from 20 poultry farms near Tehran, Iran. Furthermore, recent reports about carriage of antibiotic-resistant pathogens by M. domestica collected from different fast-food restaurants in northern Kansas (USA) [8] and in hospitals of several countries of Sub-Saharan Africa [9] are quite alarming and calls for urgent M. domestica control measures.
The ENPFs are significant biological control agents against M. domestica due to their low mammalian toxicity and natural prevalence among flies population. However, compared to literature reports on mosquito control, the studies for M. domestica control is significantly few and far between. The literature studies describing M. domestica control using ENPFs have mostly used E. muscae, B. bassianaand M. anisopliae. Most of these studies pertain to control of adult fly [10-12]; while few of these reports have explored control of M. domestica larval stage [12,13]. These studies indicated absolute mortality of M. domestica in 5-15 days. However, a substantial part of these studies has been conducted in temperate region, where the control agents have tried to mimic the prevalent environmental conditions. The research works carried out in the temperate region with local fungal strains have little significance in tropical/sub-tropical conditions of Indian subcontinent. The reason for this partly lays in lack of adaption of fungal strains towards the varying temperature, humidity and soil conditions of tropical/subtropical region. Furthermore, pathogenicity of ENPFs is strain dependent and is influenced by geographical conditions [14]. Kryukov et al. [14], reported high variability in virulence of B. bassiana cultures isolated in a small territory or even at the same site, due to formation of the fungal races adapted to kill local insect populations or specific hosts. Adaptability of ENPF strains to a particular environment may also increase its infectivity by affecting their genetic polymorphism [15]. Considering the above facts, the investigation of native ENPF isolates adaptable to local environment and hence more efficient for the control of pest population of the region is desirable, to ensure the production of biopesticides comparable to conventionally used chemical insecticides in terms of cost and activity. Thus, the present study has undertaken the screening of suitable native isolates of ENPFs for the use as biopesticidal agents against M. domestica.
Methods
Collection and analysis of soil sample
Soil samples were collected from different fruit orchards in northern India during september-november, 2009. A total of 8 fruit orchards were sampled. Soil samples were taken using a cylindrical soil corer with an inner diameter of 5 cm. From each orchard, 10 randomly selected soil cores (diameter-5 cm, depth-5 cm) were removed and placed as subsamples in a plastic bag. Collected soil samples were analysed for carbon and nitrogen content through CHN analyzer (VARIO EL III). The pH of the soil solutions was analysed by a digital type pH meter (HI 2212 pH meter, Hannah instruments, USA) by dissolving 1 g of soil in 10 ml of distilled water.
Isolation of fungal strains
Isolation of ENPF from soil was done by Galleria bait method. 100 g of air dried and re-moistured soil samples were placed in cylindrical plastic bottle (90 mm × 140 mm) along with 10 pre heat treated larvae of Galleria mellonella (Lepidoptera: Pyralidae). The heat treatmentprevents webbing by G. mellonella larvae [16]. The bottles were sealed with perforated lids and incubated at 22°C ± 1°C and 65% relative humidity (RH) in a growth chamber for 16-18 days. During the incubation for first week, containers were inverted to make the vigorous bait insects penetrate maximum amount of soil [16]. Containers were examined regularly for the dead larvae, which were collected and surface-sterilized with 1% sodium hypochlorite solution for 3 min, followed by gentle rinsing (2 times) with distilled water. Dead larvae were placed on Petri plate containing selective medium [1% peptone, 2% glucose and 1.2% agar along with 0.5 ml of 0.6 g/ml Streptomycin, 0.5 ml of 0.05 g/ml Tetracycline, 0.5 ml of 0.1 g/ml Dodine and 2.5 ml of 0.05 g/5 ml Cyclohexamide] for isolation of ENPFs. The plates were incubated at 27°C and 100% RH in a growth chamber and observed for the presence of fungi. The fungi were sub-cultured to obtain pure cultures and maintained on potato dextrose agar (PDA) slants.
Growth pattern, spore production, biomass and spore hydrophobicity
Fungal isolates obtained through isolation from soil samples were morphologically characterized using culturing on artificial media; potato dextrose agar (PDA) on Petri plates and potato dextrose broth (PDB) in culture flasks. Both the type of media were inoculated with 1 ml of conidial suspension (1 × 106 conidia/ml) of different fungal isolates and incubated at 28°C for 8 days under stationary (PDA) or as shake cultures at 180 rpm (PDB). The growth of each fungal strain was studied in terms of colony morphology (color and growth pattern), and radial growth of isolates. Sporulation was recorded by excising 20 mm2 colony area from fungal growth on PDA medium using a cork borer, followed by mixing with 1 ml of distilled water to dislodge spores [17]. The spores present in the sample were counted by Automatic Cell Counter (Cellometer® Vision HSL, Nexcelom Bioscience). Biomass production was assessed from fungal growth on PDB media. After the incubation period, the dry cell weight of the biomass was estimated by filtering out the contents of flasks through predried and preweighed Whatman no. 1 filter paper, and drying it at 54°C to a constant weight.
The spore hydrophobicity of fungal isolates was determined by the method described by Shan et al. [18] For each isolate, spores were suspended in PM buffer (per liter: K2HPO4, 6.97 g; KH2PO4, 2.99 g; MgSO4.7H2O, 0.2 g) containing 0.02% (v/v) Tween 80 at pH of 7.12. The spore suspension was standardized to 2 × 106 spores/ml, followed by addition of 0.1% liquid paraffin. The mixture was vortexed for 2 min to separate organic phase from the aqueous phase. The spores in the aqueous phase were counted using an Automatic Cell Counter. The hydrophobicity index (HI) of the tested spores of fungus isolates was determined using following formulae:
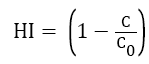
where C0 and C is the spore concentration of the aqueous phase before and after the addition of liquid paraffin, respectively. The assay was repeated thrice for each isolate.
Microscopic observation of fungal isolates
Microscopic observation of fungal isolates was done using phase contrast microscope (Leica Microscope; Magnification: 40X) and scanning electron microscope (SEM). For SEM analysis, 10 μl inoculum of fungal growth in liquid culture was primarily fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer for 1-2 h. Thereafter, it was washed with distilled water for 10-20 min, followed by secondary fixation with 1%-4% osmium tetroxide (OsO4) in distilled water for 1-2 h. Secondary fixation was followed by washing with distilled water (10-20 min) and then serial dehydration with 45%, 60%, 75%, 90%, and 100% ethanol for 10 min each. After dehydration, samples were subjected to critical point drying (CPD) in hexamethylene disilazane (HMDS). All the processes were performed at room temperature. Samples were mounted on silver stub for SEM observation and gold-covered by cathodic spraying (Polaron gold). Observations were made on a scanning electronic microscope (ZEISS EVO 50) under the following analytical condition: EHT ¼ 20.00 kV, WD ¼ 9.5 mm, Signal A ¼ SE1.
Insecticidal activity of fungal isolates against M. domestica
Insecticidal activity of fungal isolates was determined in a Petri plate bioassay against M. domestica adult and larvae according to the method described earlier by Mishra et al. [1] Fungal dose used for the assays was 1 ml of conidial suspension in 0.1% Tween 80 at 1 × 108 spores/ml. Control was sprayed with 1 ml of sterile distilled water containing 0.1% Tween 80. Treated plates were incubated at 28 ± 5°C, 65% RH and 14:10 photoperiod. For each treatment, four replications were incorporated, while whole set up was repeated thrice. For assay against M. domestica adults, lab reared flies (10 in no., 2-3 days old) were placed on a filter paper (Whatman no. 1) in Petri plate (diameter-150 mm, area=17662.5 mm2, BOROSIL®) containing diet consisting of mixture of groundnut oil cake and wheat bran in 1:3 ratios. Dead adult flies were counted daily during five days of monitoring. Fungal infection on dead flies was confirmed by incubation of surface sterilized dead cadaver on PDA media, followed by incubation at 28 ± 5°C, 65% RH, for allowing growth of fungus on cadaver. For larval assay, a batch of 20 lab reared larvae (2nd in star) of M. domestica was taken on Petri plates (diameter-90 mm) along with larval diet as described previously [1]. The mortality of M. domestica larvae was adjudged by larval wasting and immobility, monitored daily for five days [1]. Mortality data were corrected for control mortality using Abbott’s [19] formula and normalized using arcsine transformation before being subjected to a two-way factorial analysis of variance using Stat Plus [20]. The means were separated using LSD and differences between them were considered significant at P<0.05.
The fungal isolates obtained in the present study were compared for their insecticidal activity with twelve different isolates of ENPF procured from microbial type culture collection (MTCC) and national bureau of agriculturally important insects (NBAII), India (Table 1). Selection of isolates for procurement was made on the basis of literature reports for their insecticidal activity against various insect/pests. The procured isolates were investigated for their insecticidal activity against M. domestica adults and larvae according to the method described above.
| S. no. | Fungus isolate | Accession no. | Strain | Host | Source |
|---|---|---|---|---|---|
| 1 | Beauveriabassiana | MTCC 4562 | E90 | Fly | Mandla, Chattisgarh, India |
| 2 | Beauveriabassiana | MTCC 4580 | E74 | Insect | Jabalpur, Madhya Pradesh, India |
| 3 | Beauveriabassiana | MTCC 4514 | E123 | Hyblaeapuera | Hosangabad, Madhya Pradesh, India |
| 4 | Metarihiziumanisopliae | MTCC 3210 | E/98/1 | Cricket | Jorhat, Assam, India |
| 5 | Paecilomycesfumosoroseus | MTCC 4099 | E24 | Grub | Kalpi, Madhya Pradesh, India |
| 6 | Paecilomycesfarinosus | MTCC 4114 | E20 | Plusia (Insect) | Dantewara, Chattisgarh, India |
| 7 | Beauveriabassiana | PDBC-Bb-5a | - | Hypothenemushampei(Coffee berry borer) | Coorg, Karnataka |
| 8 | Verticilliumlecanii | PDBC-Vl-1 | - | Aphids of Vegetable crops | Bangalore, Karnataka |
| 9 | Metarhiziumanisopliiae | PDBC-Ma-4 | - | Grubs of Plocaederusferrugineus(Cashew Stem Borer) | Dakshina Kannada, Karnataka |
| 10 | Nomuraea riley | PDBC-Nr-44 | - | Larvae of Pierisbrassicae (Cabbage Butterfly) | Jorhat, Assam |
| 11 | Nomuraea riley | PDBC-Nr-1 | - | Larvae of Spodopteralitura from Cotton Fields | Kurnool, Andhra Pradesh |
| 12 | Paeciliomycesfumosoroseus | PDBC-P-1 | - | Grubs of Brahmina sp. from Potato fields (Potato root grub) | Solan, Himachal Pradesh |
Table 1: Entomopathogenic fungal isolates procured from Indian Culture Collection Center.
Molecular characterization of fungal isolates through RAPD-PCR amplification
Variation among different fungal isolates was further ascertained using molecular amplification method by random amplified polymorphic DNA (RAPD). For this, DNA was isolated from fungal isolate using freeze drying of fungal mycelia, which was further extracted by CTAB method [17]. Quantity of DNA was estimated by spectrophotometer while its quality analysis was done by 0.8% agarose gel electrophoresis. For RAPD analysis, twenty RAPD primers (obtained from Bangalore Genei™) were screened, of which twelve gave amplification, however only six primers gave scorable and reproducible results. Polymer chain reaction (PCR) amplification reaction for RAPD analysis was carried out in the final volume of 25 μl [1 × assay buffer (2.5 μl), 10 mM dNTPs (1.25 μl), 3U Taq DNA Polymerase (1 μl), 2.5 mM MgCl2 (1.25 μl), 25 ng DNA template (5 μl), 20 μM primer (2 μl)]. Amplifications were executed using an initial step of 15 s at 94°C, followed by 45 cycles (94°C at 15 s, 36°C at 30 s, 72°C at 1 min), and a 7 min final extension at 72°C. RAPD data obtained was cluster analysed to examine the genotypic relationship among the isolates. Amplification products were scored as either present (1) or absent (0). Numerical analysis was done using NTSYS PC version 2.0. A data matrix was prepared to determine similarities between each pair of genotype. Similarities were calculated using simple matching coefficient and clustering was achieved by unweighted pair group method using arithmetic averages (UPGMA).
18s-rRNA identification of selected isolate
Based on the insecticidal activity data, the isolate with maximum pathogenic potential was selected for molecular identification through 18s-rRNA analysis. The process accompanied DNA isolation followed by amplification reaction. PCR amplification was performed with 3 primers; primers P1 (5′-AAGCTTCGACATGGTCTG-3′), ITS1 (5′- TCCGTAGGTGAACCTGCGG-3′) and I21 (5′- CGATCCTTTAGTCCCTCGAC-3′), chosen on the basis of literature report for molecular characterization of Beauveria strains [21,22]. PCR amplification reaction was carried out in the final volume of 25 μl [1 × assay buffer (2.5 μl), 10 mM dNTPs (1.25 μl), 3U Taq DNA Polymerase (1 μl), 2.5 mM MgCl2 (1.25 μl), 25 ng DNA template (5 μl), 20 μM primer (2 μl)]. The enzymatic amplification was performed in a thermo cycler programmed for 1 cycle for 4 min at 94°C, 35 cycles: 45 s at 94°C, 45 s at 57°C and 45 s at 72°C (extension of the primer). After 35 cycles an extra extension step was performed for 4 min at 72°C. Amplified products were separated by submerged gel electrophoresis on 1.5% agarose gel in 1 × TAE buffer. The gel was visualized under UV Transilluminator attached with Kodak camera and the picture was taken with gel documentation system (Ultra.lum). PCR product obtained after amplification and purification in 12 μl of 20% PEG-NaCl (Polyethylene glycol- NaCl) solution was subjected to sequencing reactions based on dideoxynucleotide chain termination method [23]. A 2 μl PCR product and 3 pmol of the sequencing primer were used in a 20 μl sequencing reaction and subjected to 25 cycles in a Perkin Elmer thermal cycler 9700. Each cycle consisted of 95°C for 10 min, 50°C for 5 min and 60°C for 4 min. DNA sequencing was carried out on ABI 1500 automated sequencer at the DNA sequencing facility of Ocimum Biosolution (P) Ltd. The sequences obtained were in small fragments and hence it was aligned properly by overlapping the sequences. The nucleotide sequence was analyzed with the GenBank database using BLAST program [24].
Results
Collection of soil sample and Isolation of fungus isolates
Soil samples were collected from different fruit orchards and nurseries located in Northern India during September- November, 2009. Characterization of collected soil samples for selected parameters is represented in (Table 2). A total of 5 ENPF strains (later identified as strains of “Beauveria bassiana”) were isolated. The soil samples harboring ENPF showed a narrow pH range (7.2-8.5), with inclination towards alkalinity (pH>8).
| S. no. | Field type | C (%) | N (%) | pH | Entomopathogenic fungi obtained |
|---|---|---|---|---|---|
| 1 | Mango Orchard | 1.996 | 0.253 | 8.5 | Isolate 1 |
| 2 | Coriander Nursery | 1.188 | 0.183 | 8.2 | None |
| 3 | Mango Orchard 2 | 1.173 | 0.192 | 7.6 | Isolate 5 |
| 4 | Horticultural Research Centre | 0.877 | 0.166 | 7.2 | Isolate 2 |
| 5 | High density Mango Orchard | 0.865 | 0.15 | 8.4 | Isolate 3 |
| 6 | Rose Nursery | 0.788 | 0.171 | 8.3 | Isolate 4 |
| 7 | Aloe Vera Nursery | 0.785 | 0.153 | 8.2 | None |
| 8 | Wheat Nursery | 0.589 | 0.153 | 5.7 | None |
Table 2: Description of isolation sites for entomopathogenic fungal isolates.
Growth pattern, spore production, biomass and spore hydrophobicity
Growth of fungal isolates in different media provided information about their colony forming properties, growth pattern and vigorousity. Growth of fungal isolates on PDB medium was rapid till 3.5 days, followed by decline in growth rate which was quite visible in case of isolate ‘1’. Mycelium of different fungal isolates was white with fluffy to powdery appearance. The color of colony/or mats was observed to be white, which became yellowish white or pale pinkish (isolate ‘3’) with increase in time.
The spore production among the isolates showed significant variation (p<0.05); with yield for different fungal isolates ranging from 8.3 × 105-2.5 × 108 spores/cm2 (Table 3). The maximum spore production was observed in isolate ‘1’, while isolate ‘5’ had lowest spore yield. After an incubation period of 8 days, radial growth of fungal isolates varied between 4.2-7.7 cm (Table 3).
| Fungus isolates | Mean radial growth (cm) | Spore production (conidia/cm2) | Biomass (g/L, mean ± SD) | Hydrophobicity index (mean ± SD) |
|---|---|---|---|---|
| Isolate 1 | 7.7 | 2.5 × 108 | 5.66 ± 0.191 | 0.795 ± 0.016 |
| Isolate 2 | 4.8 | 3.8 × 107 | 5.23 ± 0.163 | 0.542 ± 0.009 |
| Isolate 3 | 5.4 | 7.2 × 106 | 6.53 ± 0.237 | 0.589 ± 0.011 |
| Isolate 4 | 7.3 | 2.7 × 106 | 3.04 ± 0.091 | 0.822 ± 0.021 |
| Isolate 5 | 4.2 | 8.3 × 105 | 1.35 ± 0.086 | 0.558 ± 0.013 |
Table 3: Mean radial growth, Spore production, Biomass and Hydrophobicity index of different fungal isolates.
The maximum mean radial growth (7.7 cm) was observed in isolate ‘1’, followed by isolate ‘4’ (7.3 cm). Isolate ‘5’ showed least radial growth at 4.2 cm. Radial growth of the fungal isolates represents the frequency of their hyphal branching and substrate affinity. The biomass production with different isolates varied between 1.35-5.66 g/L, with maximum yield from isolate ‘1’ (Table 3). The biomass and spore yield for different fungal isolates is indicative of their pathogenicity. Hydrophobicity index of fungal spores varied significantly (F=850.3; df=4; p<0.001) amongst different fungal isolates ranging from 0.54-0.82 (Table 3). The spores of isolate ‘4’ were found to be most hydrophobic with hydrophobicity index of 0.82, followed by that of isolate ‘1’ (0.79).
Microscopic observation of fungal isolates
Microscopic observation of fungal isolates by phase contrast microscopy is represented in (Figure 1). Mycelial strand of the fungal isolates were observed to be cylindrical, hyaline and septate. Isolate ‘1’ revealed oval to round spores, whereas spores of isolate ‘2’ were oval and relatively larger in size. Isolate ‘3’ had flask shaped spores. The shape of spores for isolate ‘4’ and isolate ‘5’ was round and globose to subglobose, respectively. The spore shape of fungal isolates gave an indication that these isolates belongs to Genus “Beauveria” [25]. The sporogenous cells formed tightly clustered groups, appearing as fluffy balls within the aerial hyphal mass.
Figure 1: Phase contrast microscopic image of fungal isolates; A: Isolate 1; A1: enlarged image of fungal hyphae of isolate 1, B: Isolate 2, B1: enlarged image of fungal spores of isolate 2, C: Isolate 3, D: Isolate 4, D1: enlarged image of fungal hyphae of isolate 4, E: Isolate 5 [Magnification 40X].
The SEM image for different fungal isolates revealed narrow and septate hyphae (Figure 2). The vesicles generally formed globose type of structure, with size varying between 1.5 μm-5 μm for different fungal isolates. Majority of spores of fungal isolates showed globose to oval shape, while their arrangement was basifugal. These features provided further evidence that the fungal isolates belongs to Genus “Beauveria”. Further characterization of fungal isolates for morphological identification was carried out by indian type culture collection (ITCC), Indian Agricultural Research Institute (New Delhi), which confirmed the fungal isolates to be strains of Beauveria bassiana.
Insecticidal activity of fungal isolates against M. domestica
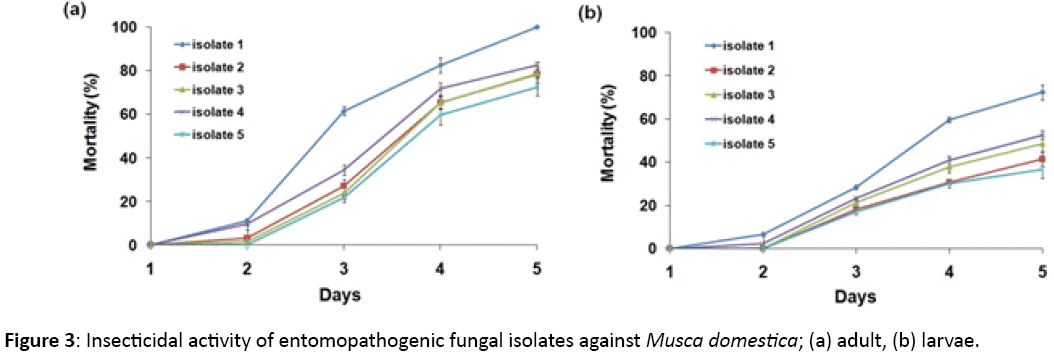
The mortality data for M. domestica adults and larvae showed significant variation among different isolates (p<0.001). Significant results were also obtained for mortality data recorded between different observation periods for fungal isolates (p<0.01). For M. domestica adults, isolate ‘1’ showed 100% mortality, followed by 82.3% mortality obtained with isolate ‘4’, after 5 days of observation (Figure 3a). At 24 h, no mortality was shown by any of the Beauveria isolates, which increased to 0%-11% after 48 h, and 21.8%-61.6% post 72 h of observation. After 96 h, mortality data for different fungal isolates varied between 59.8%-82.6%. The mortality of M. domestica larvae varied between 36%-72.3%, with Isolate ‘1’ resulting in maximum 72.3% larval mortality (Figure 3b). Different Beauveria isolates showed no larval mortality after 24 h, while 0%-6.4% mortality was achieved 48 h post observation. After 72 h of observation, 17.1%-28.2% larval mortality was revealed, which increased to 30.0%-59.7% after 96 h of observation (Table 4) represents the statistical interpretation of mortality data with different isolates of Beauveria. The data from (Table 4) shows the coefficient of determination (R2) for different isolates to be close to 1 (0.920-0.961) indicating a high correlation between observed and predicted values, while linear regression equation indicates absolute mortality of inst for different observation days.
| Statistical values | M. domestica adult | M. domestica larvae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolate 1 | Isolate 2 | Isolate 3 | Isolate 4 | Isolate 5 | Isolate 1 | Isolate 2 | Isolate 3 | Isolate 4 | Isolate 5 | |
| R Square | 0.956 | 0.939 | 0.924 | 0.961 | 0.92 | 0.961 | 0.949 | 0.947 | 0.961 | 0.942 |
| Adjusted R Square | 0.942 | 0.919 | 0.899 | 0.948 | 0.893 | 0.948 | 0.932 | 0.929 | 0.948 | 0.922 |
| Standard Error | 10.587 | 10.11 | 11.533 | 8.319 | 11.02 | 7.307 | 4.791 | 5.849 | 5.292 | 4.693 |
| Linear Regression equation | Isolate 1 =-30.4400 + 27.1600 *Days | Isolate 2 =-30.7800 + 21.8867 *Days | Isolate 3 =-32.4200 + 22.0933 *Days | Isolate 4 =-28.4900 + 22.7300 *Days | Isolate 5 =-30.6067 + 20.4800 *Days | Isolate 1 =-26.0567 + 19.8033 *Days | Isolate 2 =-15.9600 + 11.3467 *Days | Isolate 3 =-18.9833 + 13.5233 *Days | Isolate 4 =-19.3700 + 14.4033 *Days | Isolate 5 =-14.2600 + 10.3400 *Days |
Table 4: Statistical interpretation of mortality kinetics for M. domestica adult and larvae.
Insecticidal activity of procured isolates of ENPF is represented in (Figure 4). Amongst the procured isolates, B. bassiana NBAII Bb5a was found to be most effective showing 97.7% mortality of M. domestica adults within 5 days (Figure 4a). For the same period of observation, Metarhizium anisopliae Ma4 and M. anisopliae MTCC 3210 with a mortality of 94.72% and 92.72%, respectively, also showed high efficacy for control of adult flies. The M. domestica mortality varied between 73.7%-97.7% with different strain of B. bassiana. The different strains of Nomuraea riley (86.7%-90.1% mortality) and Verticillium lecanii (88.7% mortality) were also found to be very effective control agent against M. domestica. The strains of Paecilomyces fumosoroseus (77.1%-80.4%) were moderately effective against M. domestica adults, while P. farinosus (64.1%) was found to be least effective control agent. Overall, within the procured fungal strains, isolates of M. anisopliae were observed to be most effective for the control of adult flies. However, comparing the mortality data of procured fungal isolates with Isolate ‘1’, Isolate ‘1’ with an absolute mortality within 5 days was more effective than any of the procured fungal strains.
Figure 4b shows the Insecticidal activity of procured ENPFs against M. domestica larvae. The fungal isolates showed low larval mortality, varying between 3.3%-23.3%. Different strains of B. bassiana with larval mortality between 3.3%-16.7% were poor performer, while the strains of M. anisopliae (20%-23.3% mortality) and V. lecanii (16.7% mortality) showed maximum larval mortality. In comparison, the native strains of B. bassiana isolated in the present study showed much higher larval mortality (36%-72.3%). Since, procured ENPFs did not perform well in terms of direct larval mortality; another parameter was used to study their sondary impact. This was calculated as percentage suppression in emergence of adult from the pupae formed from the treated larvae. This parameter indicates the carryover of infection in the pupal and adult stage. Compared to the mortality data, various fungal treatments showed better results for suppression in adult emergence. B. bassiana NBAII Bb5a with a maximum 44.4% suppression in adult emergence was found to be best performer, followed by M. anisopliae Ma4 (39.1% suppression in adult emergence). The fungal isolates, P. farinosus MTCC 4114 (14.8%) and P. fumosoroseus NBAII P1 (17.2%) were least effective in suppressing emergence of adult fly. Thus, M. anisopliae Ma4 with a total activity (summation of mortality and adult emergence suppression) of 62.5% was regarded as most effective control agent amongst procured fungal strains. The ENPFs, B. bassiana Bb5a and M. anisopliae MTCC 3210 with a total activity of 54.4% and 40.8%, respectively, showed moderate efficacy.
Molecular characterization of fungal isolates through RAPD-PCR amplification
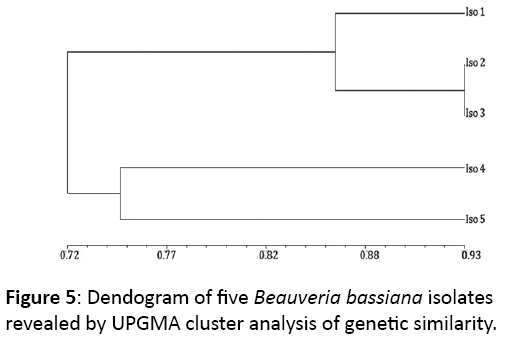
RAPD analysis using 12 random primers produced six scorable bands with reproducible results. Jaccard’s coefficient of similarity was generated from RAPD analysis data of B. bassiana isolates and values obtained were subjected to UPGMA cluster analysis of genetic similarity to create a dendogram (Figure 5). Dendogram of the isolates showed maximum similarity between Isolate 2 and 3. Isolate ‘1’ was found to be most similar to isolate ‘3’, while least similar to isolate ‘5’.
18s-rRNA identification of selected isolate
Isolate ‘1’ depicting best adulticidal and larvicidal activity, was taken up for molecular identification using 18S rRNA-PCR method. The genomic DNA of selected B. bassiana isolate (isolate ‘1’) when run in 0.8% agarose gel was found to be intact and of high molecular weight. The spectrophotometric analysis of the DNA showed that the ratio of absorbance at 260 nm vs 280 nm was 2 indicating that the preparation was pure and free from protein contamination. The PCR product of 18S rDNA was amplified as a single band through three different primer set (Figure not shown). The PCR products were purified and sequenced in automated sequencer using universal primers. The sequences obtained from each set of forward and reverse primer were then overlapped to get 1101 bp 18S rDNA sequence. The 18S rDNA and ITS nucleotide sequence were analyzed with the GenBank database using BLAST program. The 18S rDNA sequence showed 99% homology with 18S rDNA of B. bassiana. The nucleotide sequences of 18S rDNA formed new isolate of B. bassiana and have been deposited in NCBI GenBank database. The accession numbers assigned for the isolate was HQ917687.
Discussion
In order to compete with the conventionally used chemical insticides, investigation of native ENPF isolates which are adaptable to the local environment and hence more efficient for the control of pest population of the region, is desirable. The present study with an attempt to explore native isolates of ENPFs from Indian Subcontinent isolated and characterized five isolates of B. bassiana from soil samples. Majority of the isolates were obtained from the soil with pH ranging between 8.2-8.5. The similar findings were observed by Quesada- Moraga et al. [26], who reported greatest percentage of fungal isolation in the soil samples with pH value 8–8.5. On the contrary, Ali-Shtayeh et al. [27], reported minor effect of pH on ENPF abundance in the cultivated soils. The present study showed high occurrence of ENPF in the soil containing higher carbon content. However, one exception was coriander nursery from where no ENPF isolate could be obtained in spite of high carbon content in the soil. This could be attributed to the presence of aromatic compounds in coriander plant, which is supposed to negatively affect fungal occurrence. This reflects the effect of vegetation type on the presence of ENPFs in a habitat. The present study also showed higher occurrence of ENPF in orchards compared to the nurseries. Similar findings were also observed by Ali-Shtayeh et al. [27], who reported higher percentage of ENPF isolates from orchards soils. Sun et al. [28], reported high frequency of M. anisopliae var. anisopliae and B. bassiana in crop field and orchard soils, respectively. Ali-Shtayeh et al. [27], postulated that lack of fungal diversity at a particular site does not necessarily mean low number of infective propagules for that fungal species. In their study, it was demonstrated that the soil biota in Palestine had low diversity of ENPF with only few species occurring frequently. The present study had similar observation with occurrence of single ENPF isolate from a particular site, depicting lack of diversity. The isolation of single fungal genera may have been favored by their persistence and occurrence of large number of infective propagules, supporting selection of a particular species.
The B. bassiana isolates in the present study showed wide variation in colony color, spore and biomass production. Similarly, Varela and Morales [29] reported wide variation (between white and yellow) in colony color of different B. bassiana isolates. Liu et al. [30], reported variation in spore production (1.6 × 106-1.56 × 107 spores/cm2) for the four isolates of B. bassiana, with the most virulent isolate producing significantly larger number of spores. The value of hydrophobicity index denotes interfacial free energy for interaction [31]. This value represents the degree to which the attraction of the cells to water is greater or smaller than the attraction of water molecules to each other [32]. The lower value for this free energy of interaction makes the molecule hydrophilic which then tend to disperse in water, while the larger value indicate hydrophobicity of the entity resulting in particles aggregation in aqueous solution. Fungal spores with hydrophobicity index >0.7 have been reported to be hydrophobic [32]. These authors also reported aerial spores of B. bassiana to be most hydrophobic, and thus likely to aggregate in water. Earlier, Shan et al. [33], reported hydrophobicity rate of B. bassiana aerial spores to be between 69.5%-87.2%. In the present study, aerial spores of isolate ‘1’ and ‘5’ showed high hydrophobicity, while spores of other B. bassiana isolates were less hydrophobic. Higher hydrophobicity is a complementary attribute, favoring adherence of spores to the host surface and thus augmenting fungal virulence.
Microscopic characterization of B. bassiana isolates in the present study showed a variable degree of hyphal and spore polymorphism. The polymorphism of spores observed in different fungal isolates confers to their genetic variability [29]. Beauveria species are classified by the shape of their spores and their placement of on sporogenous apparatus. Mugnai et al. [15] reported spore shapes of fungi as the most useful single character out of 64 morphological and biochemical tests. In their study, spore shape of B. bassiana was found to be heterogeneous, while B. brongniartii isolates was shown to produce ellipsoidal or cylindrical spores. Glare and Inwood [34] reported occurrence of globose, spherical and ellipsoidal spores in B. bassiana strains from New Zealand. In their study, sporogenous cells of B. bassiana formed tightly clustered groups with swollen base and irregularly bent rachis. The shape of spores may vary even for the same fungal strain depending upon the substrate type as illustrated by Townsend et al. [35] The Beauveria isolates (e.g., F185 and F186) in their study, produced ellipsoidal and spherical spores on the inst host and artificial culture medium, respectively. In a different study, the fungal strains with a spore length >3 μm and ratio of spores length to width ≥ 2have been reported to be B. brongniartii or one of the other Beauveria species [34]. Significant differences in spore size, production, speed of germination and relative hyphal growth among the different B. bassiana isolates was also reported by Liu et al. [30]
There have been few studies evaluating the efficacy of ENPFs for M. domestica control. In this regard, present study showing appreciable pathogenic activity of all 5 native B. bassiana isolates against adultand larvae life stages of M. domestica was commendable. The earlier studies on the pathogenic activity of B. bassiana against M. domestica reported variation in activity for different strains, similar to the observation in the present study [10,35]. Barson et al. [13], observed 12%-100% (B. bassiana 061345) and 25%-77% (B. bassiana 12943) mortality of adult M. domestica, whereas Watson et al. [36] noted significant fly mortality (8.5%-99.3%) with different strains of B. bassiana. Similarly Lecuona et al. [11], reported 87.7%-98.0% mortality of adult M. domestica with different isolates of B. bassiana (Bb 72, 10, 6, 96, 4). Absolute mortality of adult flies in these literature studies was reported at 5 to 7 days post-exposure. Barson et al. [13], reported 2%-100% mortality of adult flies with B. bassiana 061345 after 6 days of treatment, which increased to 12%-100% at 9 days post exposure. In the study by Siri et al. [10], B. bassiana application resulted in 94% adult mortality after 14 days of exposure. The only study retrieved for larvicidal action of B. bassiana against M. domestica larvae by Barson et al. [13], reported 40% larval mortality after 14 days of B. bassiana (isolate no.-061345) treatment. Compared to the above studies, the absolute mortality of M. domestica adults post 5 days exposure as obtained in the present study was significant improvement. As far as larvicidal activity is concerned, present study makes an important contribution with 72% larval mortality. The result also establishes that the native isolates were generally better M. domestica control agents.
RAPD has been successfully used to distinguish pathogenic and non-pathogenic isolates of Leptosphareia maculans [37] and to differentiate pathotypes in Peronospora parasitica [38]. RAPD analysis has also been reported to show a certain correlation between B. bassiana isolates and their geographical origin [38]. In the present study, RAPD primers were used to observe polymorphism within the B. bassiana isolates. Thus molecular markers were able to distinguish between the isolates within the same group, while the cluster analysis revealed a complex pattern of association among the isolates. In the present study, results obtained from RAPD were in conformity with various other properties of isolates assessed earlier. According, to RAPD analysis, isolate ‘2’ was most similar to isolate ‘3’, while isolate ‘1’ and ‘5’ showed maximum variation between them. Similar relation was obtained between isolates during assessment of their biological properties, where biomass and spore production from isolate ‘2’ and ‘3’ was nearly similar, while significantly varied for isolate ‘1’ and ‘5’. Similarly, hydrophobicity index for spores of isolate ‘1’ (0.79) and ‘5’ (0.56) showed wide variation. The Insecticidal activity of different fungal isolates followed similar trend, where isolate ‘2’and‘3’ showed comparable activity against adults (78.3% and 78.7%, respectively), and larvae (41.3% and 48.7%, respectively). Analogously, isolate ‘1’ and ‘5’ showed extensive dissimilarity for their Insecticidal activity. The variations between fungal isolates depicted through various morpho-molecular characterizations and pathogenecity assessment reflected their strain diversity. The results also signified the importance of strain selection for effective control measures as well as for further consideration of commercial aspects.
Conflict of Interest
Authors’ declare that the present research work does not have any kind of financial, academic, commercial, political or personal conflicts with any institution or individual.
Acknowledgment
This paper is dedicated to the memory of Late, Padma Vibhusahn, Dr V P Sharma, Founder Director of National Institute of Malaria Research (India) & Former Additional Director General, Indian Council of Medical Research, who inspired the authors constantly with valuable suggestions on insect pest/vector control. Financial support by Indian Council of Medical Research (IRIS_ID No. 2010-07860), India and CSIR Research Associateship (09/086 (1191)/2014-EMR-I) to one of the author (SM) is gratefully acknowledged. The authors also acknowledge Mr. Satendar Singh (IIT Delhi, India) for his help in experimental work.
References
- Mishra S, Kumar P, Malik A, Satya S (2011) Adulticidal and larvicidal activity of Beauveriabassiana and Metarhiziumanisopliae against housefly Muscadomestica (Diptera: Muscidae) in laboratory and simulated field bioassays. Parasitol Res 108: 1483-1492.
- Shah PA, Pell JK (2003) Entomopathogenic fungi as biological control agents. ApplMicrobiolBiotechnol 61: 413-423.
- Goettel MS, Eilenberg J, Glare T (2005) Entomopathogenic fungi and their role in regulation of insect populations. ComprMol Insect Sci 6: 361-406.
- Malik A, Singh N, Satya S (2007) House fly (Muscadomestica): a review of control strategies for a challenging pest. J Environ Sci Health B 42: 453-469.
- Scholte EJ, Knols BG, Samson RA, Takken W (2004) Entomopathogenic fungi for mosquito control: a review. J Insect Sci 4: 19.
- Barro N, Aly S, Tidiane OC, Sababénédjo TA (2006) Carriage of bacteria by proboscises, legs, and feces of two species of flies in street food vending sites in Ouagadougou, Burkina Faso. J Food Prot 69: 2007-2010.
- Barin A, Arabkhazaeli F, Rahbari S, Madani SA (2010) The housefly, Muscadomestica, as a possible mechanical vector of Newcastle disease virus in the laboratory and field. Med Vet Entomol 24: 88-90.
- Macovei L, Zurek L (2006) Ecology of antibiotic resistance genes: characterization of enterococci from houseflies collected in food settings. Appl Environ Microbiol 72: 4028-4035.
- Boulesteix G, Le Dantec P, Chevalier B, Dieng M, Niang B, et al. (2005) [Role of Muscadomestica in the transmission of multiresistant bacteria in the centres of intensive care setting in sub-Saharan Africa]. Ann FrAnesthReanim 24: 361-365.
- Siri A, Scorsetti AC, Dikgolz VE, Lastra CCL (2005) Natural infection caused by the fungus Beauveriabassiana as a pathogen of Muscadomestica in the neotropic. BioControl 50: 937-940.
- Lecuona RE, Turica M, Tarocco F, Crespo DC (2005) Microbial control of Muscadomestica (Diptera: Muscidae) with selected strains of Beauveriabassiana. J Med Entomol 42: 332-336.
- Sharififard M, Mossadegh MS, Vazirian-zadhe B, Mahmoudabadi AZ (2011 Laboratory pathogenicity of ENPF Beauveriabassiana (Bals) Vuill. and Metarhiziumanisopliae (Metch.) Sorok. to larvae and adult of housefly Muscadomestica L (Diptera: Muscidae). Asian J BiolSci 4: 128-137.
- Barson G, Renn N, Bywater AF (1994) Laboratory evaluation of six species of entomopathogenic fungi for the control of the house fly (Muscadomestica L.), a pest of intensive animal units. J InvertebrPathol 64: 107-113.
- Kryukov VY, Yaroslavtseva ON, Levchenko MV, Lednyov GR, Glupov VV (2010) Phenotypic variability of environmental isolates of the entomopathogenic fungus Beauveriabassiana. Microbiol 79: 265-269.
- Mugnai L, Bridge PD, Evans HC (1989) A chemotaxonomic evaluation of the genus Beauveria. Mycol Res 92: 199-209.
- Meyling NV Methods for isolation of ENPF from the soil environment.
- Kumar P, Anshu V, Kumar S (2011) Morpho-pathological and Molecular Characterization of Bipolarisoryzae in Rice (Oryzae sativa). J Phytopathol 159: 51-56.
- Abbott WS, Dawidar, AbdElaziz M, Mortada, Mohamed M (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18: 265-267.
- StatPlus (2007) Professional build 4.9.0.0. AnalystSoft.
- Hegedus DD, Khachatourians GG (1996) Identification and Differentiation of the Entomopathogenic Fungus Beauveriabassiana Using Polymerase Chain Reaction and Single-Strand Conformation Polymorphism Analysis. J InvertebrPathol 67: 289-299.
- White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds.) PCR Protocols: A Guide to Methods and Applications. Academic Press New York, pp. 312–322.
- Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. ProcNatlAcadSci U S A 74: 5463-5467.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389-3402.
- Capek A, Fassatiová O (1977) Some biochemical characteristics of species of the genus Beauveria. Folia Microbiol (Praha) 22: 308-310.
- Quesada-Moraga E, Navas-Cortés JA, Maranhao EA, Ortiz-Urquiza A, Santiago-Alvarez C (2007) Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol Res 111: 947-966.
- Ali-Shtayeh MS, Mara'i AB, Jamous RM (2002) Distribution, occurrence and characterization of entomopathogenic fungi in agricultural soil in the Palestinian area. Mycopathologia 156: 235-244.
- Sun BD, Yu HY, Chen AJ, Liu XZ (2008) Insect-associated fungi in soils of field crops and orchards. Crop Prot 27: 1421-1426.
- Varela A, Morales E (1996) Characterization of some Beauveriabassiana isolates and their virulence toward the coffee berry borer Hypothenemus hamper. J InvertebrPathol 67: 147-152.
- Liu H, Skinner M, Brownbridge M, Parker BL (2003) Characterization of Beauveriabassiana and Metarhiziumanisopliae isolates for management of tarnished plant bug, Lyguslineolaris (Hemiptera: Miridae). J InvertebrPathol 82: 139-147.
- Strevett KA, Chen G (2003) Microbial surface thermodynamics and applications. Res Microbiol 154: 329-335.
- Holder DJ, Kirkland BH, Lewis MW, Keyhani NO (2007) Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 153: 3448-3457.
- Shan LT, Wang ZL, Ying SH, Feng MG (2010) Hydrophobicity-Related protein contents and surface areas of aerial conidia are useful traits for formulation design of fungal biocontrol agents. Mycopathol 169: 483-494.
- Glare TR, Inwood AJ (1998) Morphological and genetic characterisation of Beauveria spp. from New Zealand. Mycol Res 102: 250-256.
- Townsend RJ, Glare TR, Willoughby BE (1995) The fungi Beauveria spp. causes grass grub population collapse in some Waikato pastures. Proc 48th N Z Plant Protection Conf 48: 237-241.
- Watson DW, Geden CJ, Long SJ, Rutz DA (1995) Efficacy of Beauveriabassiana for the controlling of the housefly and Stable fly (Diptera: Muscadiae). Biol Control 5: 405-411.
- Plummer TH, Tarentino AL, Hauer CR (1995) Novel specific oglycosylation of secreted Flavo bacterium meningosepticum proteins. J BiolChem 270: 13192-13196.
- Tham FY, Lucas JA, Wilson ZA (1994) DNA fingerprinting of Peronosporaparasitica, a biotrophic fungal pathogen of crucifers. TheorAppl Genet 88: 490-496.
- Valderrama FAM, Cristancho AMA, Cháves CB (2000) Análisis de la variabilidadgenética del hongoentomopatógenoBeauveriabassiana con marcadores RAPD. RevistaColombiana de Entomología 26: 25-29.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences