Study on Biofilms of T. rubrum and T. mentagrophytes with Antifungal Susceptibility Pattern and Detection of MBL in Patients with Chronic and First Episode Dermatophytosis in a Tertiary Care Centre in South India
Veena Hemanth1, Anupma J Kindo1*, Madhu R2, Anandan Sankarasubramaian3 and Adi Krishnan3
1Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India
2Department of Dermatology, Madras Medical College, Chennai, Tamil Nadu, India
3Department of Dermatology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India
- *Corresponding Author:
- Anupma J Kindo
Department of Microbiology,
Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu,
India,
E-mail: anupamajyoti@sriramachandra.edu.in
Received date: May 11, 2022, Manuscript No. IPMMO-22-13846; Editor Assigned date: May 13, 2022, PreQC No. IPMMO-22-13846 (PQ); Reviewed date: May 24, 2022, QC No. IPMMO-22-13846; Revised date: June 3, 2022, Manuscript No. IPMMO-22-13846 (R); Published date: July 25, 2022, DOI: 10.36648/2471-8521.8.3.034
Citation: Hemanth V, Kindo AJ, Madhu R, Anandan S, Adi K (2022) Study on Biofilms of T. rubrum and T. mentagrophytes with Antifungal Susceptibility Pattern and Detection of MBL in Patients with Chronic and First Episode Dermatophytosis in a Tertiary Care Centre in South India. Med Mycol Open Access Vol.8 No.3: 34.
Abstract
Background: In India, there has been an increase in the prevalence of dermatophytosis of the glabrous skin associated with therapeutic non-response to conventional regimens and a need for prolonged duration of treatment, in the recent years. Antifungal resistance to some antifungal drugs has been observed. Biofilms produced by dermatophytes hinders the host immune response and poses difficulty in treatment. Deficiency of Mannose Binding Lectin (MBL) is known to predispose to susceptibility to infections.
Aim: To isolate dermatophytes from patients with first episode and chronic dermatophytosis and to explore the biofilm formation, and to observe their antifungal susceptibility profile; to determine the serum MBL levels and correlate with the Minimum Inhibition Concentration (MIC) values.
Methods: Phenotypic identification of the isolates done by conventional method was followed by Antifungal Susceptibility Testing (AFST) by broth microdilution method (CLSI M38-A2).
Biofilm formation assay was carried out by crystal violet staining method. ELISA based assay was performed for the samples using monoclonal antibody against the MBL carbohydrate- binding domain with reading taken at 620 nm.
Results and Conclusion
Among the 64 patients with chronic dermatophytosis and 36 with first episode, Trichophyton mentagrophytes was the most common isolate (58%) followed by T. rubrum (42%). Biofilm formation was stronger in T. rubrum (57.1%) than T. mentagrophytes (37.9%) (p<0.001). Strong biofilm was formed by 71.9% of dermatophytes causing chronic dermatophytosis as compared to none of those causing first episode, with statistical significance. Lowest MIC was seen with fenticonazole and luliconazole (0.06 μg/ml), while the highest MIC value (2 μg/ml-64 μg/ml) was for fluconazole. MBL levels were in the deficient range for both patients (0.5 ng/ml-40 ng/ml) and controls (0.048 ng/ml-0.107 ng/ml) with the higher values being observed among patients.
Keywords
Dermatophytes; Biofilm; Antifungal agents; Antifungal resistance; Trichophyton mentagrophytes species complex; Trichophyton rubrum; Mannose binding lectin protein
Introduction
The geographical distribution of dermatophytes varies depending on the environmental, socioeconomic and hygienic status of the population [1-3]. Although this infection is neither painful nor life threatening, early diagnosis and effective treatment is imperative, as it is associated with intense itching, sleep disturbance, aesthetic issues and social embarrassment resulting in impairment of quality of life. Various Indian studies have documented an increase in the prevalence of chronic/ recurrent/steroid modified dermatophytosis and shift in the etiological agent from Trichophyton rubrum to T. mentagrophytes species complex, in the last few years. Lack of compliance, inadequate duration of treatment and emergence of antifungal resistance may delay recovery. There is a need for prolonged treatment, which may result in side effects of the drugs and increased treatment cost.
Biofilms formed by microbes are highly beneficial to the organism as they act as virulence factor and evade the host immune system, with resultant increase in the antifungal resistance. These are composed of cells embedded in extracellular polymeric substances, a matrix which is generally composed of eDNA, proteins and polysaccharides. Compared to free-floating planktonic cells of the same species, biofilm pathogens can tolerate as much as 1000-fold higher levels of antimicrobial agents [4-6]. Various studies have documented the significance of biofilms in dermatophytosis. There is paucity of literature with regard to the role in formation of biofilms by dermatophytes causing infection of glabrous skin as compared to nail infection. So in this context, this study was conducted to find the etiological agents in patients with first episode and chronic dermatophytosis; evaluate the MIC of all the drugs used for treating dermatophytosis from isolates collected from various parts of south India, and to observe the ability of T. rubrum and T. mentagrophytes species complex causing infection of glabrous skin to form biofilm.
Mannose Binding Lectin (MBL) is a subfamily of proteins known as collectins (calcium–dependent collagenous lectins); the members of this family contain collagenous regions and lectin domains [7,8]. Collectin gene is located on chromosome10 [9]. There are two MBL genes: MBL-1, which is a pseudogene, and MBL-2, which encodes the MBL-2 protein. MBL is synthesized in the liver and acts as an acute phase protein. It also acts as a kind of pattern recognition receptor. Three-point mutations on the gene of MBL-2 in codons 52, 54, 57 of the first exon of the MBL gene have been shown. These mutations cause amino acid substitutions and also impair the MBL function. Studies have documented that MBL deficiency results in susceptibility to bacterial, viral, fungal and parasitic infections, and autoimmune disorders [10,11]. As not much literature is available with regard to levels of MBL in patients with dermatophytosis of glabrous skin, we attempted to evaluate the deficiency of MBL in patients with this infection.
Materials and Methods
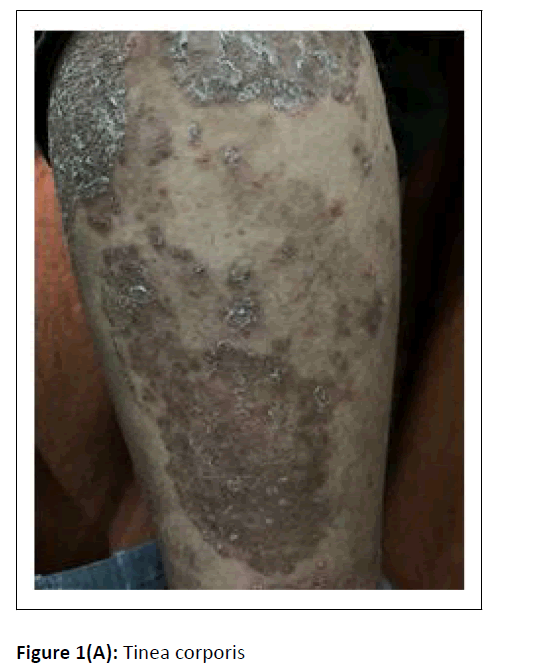
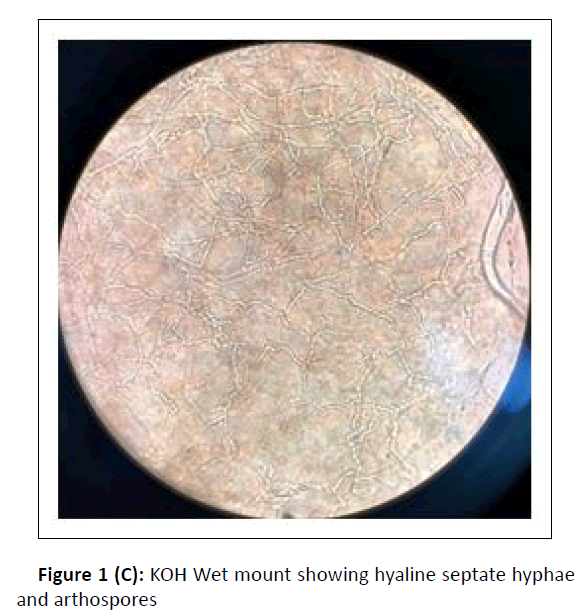
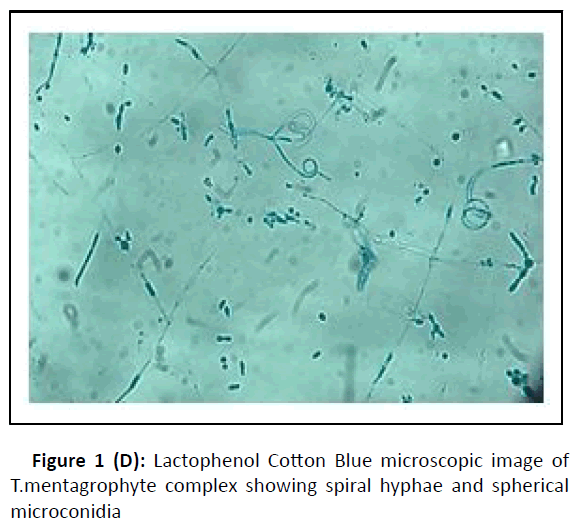
A cross-sectional study was conducted at the department of microbiology, during the period between January 2017 and February 2018. Hundred dermatophytes isolated from skin scrapings of patients with the first episode and chronic dermatophytosis, presenting with tinea corporis/tinea cruris, from three states in south India-Tamil Nadu, Kerala, and Karnataka, were included. (Chronic dermatophytosis: Presence of glabrous tinea for a duration of six months or longer, continuous or recurrent, with or without treatment.)[12] Skin scrapings were collected from the edge of the lesion with a sterile scalpel after wiping with 70% alcohol (Figure1A) and inoculated on Sabouraud Dextrose Agar (SDA) with gentamicin and actidione, and sub-cultured on Oatmeal Agar (OA) (Hi media laboratories, Mumbai, India) (Figure1B). Macroscopic and microscopic morphology were observed for speciation. (Figure1C and Figure 1D). AFST was performed by broth microdilution method (CLSI M38A2). The clinical details and susceptibility profile were noted and analyzed. The institutional ethical committee clearance was obtained (IEC-NI/16/MAR/ 51/14).
Antifungal susceptibility testing
Antifungal susceptibility of nine antifungal drugs namely fenticonazole, luliconazole, sertaconazole, itraconazole, fluconazole, voriconazole, terbinafine, griseofulvin, and amphotericin B (Sigma-Aldrich) were used. T. mentagrophytes American Type Culture Collection (ATCC MYA 4439) was included as a quality control strain. The plates were incubated at 25 °C, and the readings were taken after 4 days of incubation (Figure 1E).
Susceptibility testing was performed in duplicate and the mean MIC for each drug was chosen as the MIC value of the drug for the particular isolate. The MIC for all the drugs was taken as 80% inhibition compared to control wells except Amphotericin B wherein the MIC was taken as 100% growth inhibition.
Biofilm production and Interpretation
Biofilm was detected by tube method. A loopful of test organism was inoculated in 10 mL of trypticase soy broth with 1% glucose in test tube. The tubes were incubated at room temperature for 72 h. After incubation, tubes were decanted and washed with phosphate buffer saline (pH 7.3) and dried. Tubes were then stained with crystal violet (0.1%) and excess stain was washed with sterile distilled water. Tubes were dried in an inverted position. Visual interpretation of biofilm formation was done and it was considered positive when an adherent film lined the wall and the bottom of the tube with ring formation.
MBL ELISA: Three mL venous blood samples were collected in sterile test tubes without preservative and transferred to the mycology laboratory. Serum samples were maintained in two separate microtubes at -20 °C and an ELISA assay was performed in microwells coated with a monoclonal antibody against the MBL carbohydrate- binding domain. Bound MBL is detected with the same antibody that has been labelled with biotin, followed by development with Horseradish Peroxidase (HRP)-conjugated streptavidin and incubation with a chromogenic substrate. Reading was taken at 620 nm.
Statistical Analysis
Data was analyzed by using SPSS version 16 Statistical software. Differences between the drugs were analyzed using Friedman test and P value less than 0.05 was considered as statistical significance. Pairwise comparisons were identified by post hoc analysis with Wilcoxon signed rank test after adjusting for Bonferroni correction and significance level set at 0.003.
Results
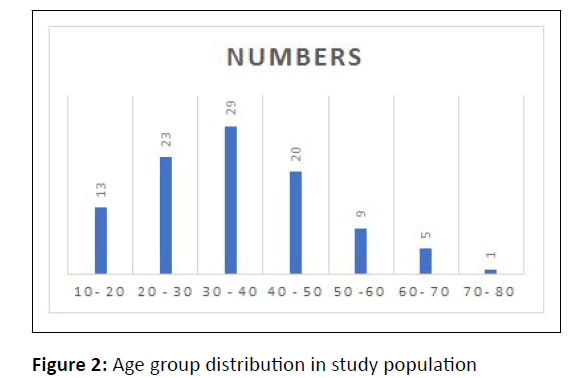
Among the 100 patients enrolled, 52% were females and 48% were males. Sex ratio was 1:1.08. Patients in the age group of 30-40 years were most affected followed by those in 20 years-30 years (Figure 2).
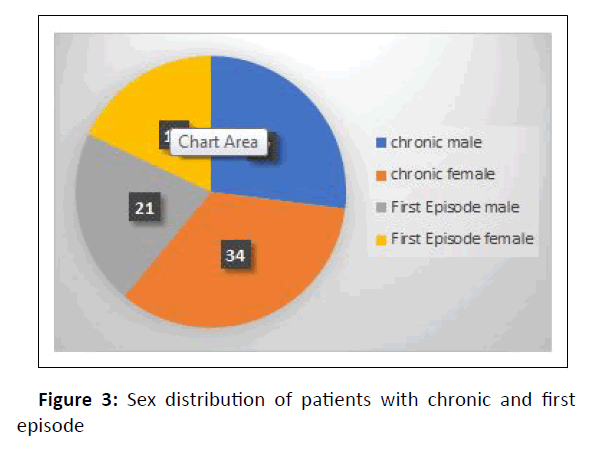
Chronic dermatophytosis was present in 64% (28 males and 36 females) of them, while 36% (15 males and 21 females) were infected for the first time (Figure 3).
T. mentagrophytes species complex was the most common organism (58%) isolated, followed by T. rubrum (42%). Among the 64 patients with chronic dermatophytosis, T. mentagrophytes complex was isolated from 36 (56.2%) and T. rubrum from 28 (43.7%) patients.
In those who came with first episode, T. mentagrophytes complex was isolated in 22 (61.1%) and T. rubrum in 14 (38.9%) patients (Table 1).
| Sl No | Etiological agent | Type of infection | |
|---|---|---|---|
| Chronic n=64 | First episode n=36 | ||
| 1 | T. mentagrophytes n= 58 | 38 (56.2%) | 20 (55.5%) |
| 2 | T. rubrum n= 42 | 26 (43.7%) | 16 (44.4%) |
Table 1: Distribution of etiological agents in chronic and first episode dermatophytosis.
Among the 100 isolates, 58 were T. mentagrophytes complex of which 22 (37.9%), 15 (25.8%) and 21 (36.2%) produced strong, moderate and weak biofilms respectively. In comparison, strong biofilm was formed by higher proportion (24,57.1%) of the total 42 T. rubrum isolates (Table 2). In the patients with first episode infection, biofilm formation was weak by 27 (75%) and moderate by 9 (25%) dermatophytes (Table 3). In contrast, among the dermatophytes causing chronic infection, 47 (73.4%) isolates were strong biofilm producers. This was followed by 13 (20.3%) and 4 (6.2%) dermatophytes producing moderate and weak biofilms respectively. Majority (26, 92.8%) of the T. rubrum isolates causing chronic infection were strong biofilm producers as compared to 58.3% (21) of T. mentagrophytes complex, which was of statistical significance (Table 4).
| Dermatophyte species | Biofilm production | No. of Isolates | Percentage % |
|---|---|---|---|
| Trichophyton mentagrophytes (n=58) | Weak | 21 | 36.2 |
| Moderate | 15 | 25.8 | |
| Strong | 22 | 37.9 | |
| Trichophyton rubrum (n=42) | Weak | 11 | 26.1 |
| Moderate | 7 | 16.6 | |
| Strong | 24 | 57.1 | |
| P value | <0.001 |
Table 2: Biofilm production by T. ment and T. rubrum.
| Biofilm production | Trichophyton mentagrophyte | Trichophyton rubrum | Total |
|---|---|---|---|
| n =20 | n=16 | ||
| Weak | 16 | 11 | 27 |
| Moderate | 4 | 5 | 9 |
| Strong | 0 | 0 | 0 |
| P value | < 0.001 |
Table 3: Biofilm production by dermatophytes causing first episode.
| Biofilm production | Trichophyton mentagrophyte | Trichophyton rubrum | Total |
|---|---|---|---|
| n =38 | n=26 | n=64 | |
| Weak | 5(13.2%) | 0 (0) | 5 |
| Moderate | 11 (28.9) | 2 (7.7%) | 13 |
| Strong | 22 (57.9) | 24 (92.3%) | 46 |
| P value | < 0.001 |
Table 4: Biofilm production by dermatophytes causing chronic infection.
MICs and Interquartile range of all the tested drugs to different dermatophytes are depicted in Tables 5 and 6. The MIC range of Terbinafine for T. mentagrophytes and T. rubrum was 0.06 μg/ml-8 μg/ml and 0.06 μg/ml-2 μg/ml respectively.
| Drugs tested (range of concentration in μg/ml) | T.mentagrophytes (n=58) μg/ml | G.Mean | T.rubrum (n=42) μg/ml | G.Mean |
|---|---|---|---|---|
| Voriconazole (32-0.125) | 0.125-4 | 0.05 | 0.06-4 | 0.067 |
| Sertaconazole (16-0.06) | 0.06-4 | 0.015 | 0.06 | 0.021 |
| Luliconazole (16-0.06) | 0.06 | 0.017 | 0.06 | 0.021 |
| Fenticonazole (16-0.06) | 0.06 | 0.017 | 0.06 | 0.021 |
| Griseofulvin (16-0.06) | 0.06-4 | 0.3925 | 0.125 - 16 | 0.619 |
| Terbinafine (16-0.06) | 0.06-8 | 0.191 | 0.06-2 | 0.076 |
| Fluconazole (64-0.25) | 2-64 | 0.221 | 2-64 | 0.1372 |
| Itraconazole (16-0.06) | 0.06-32 | 0.055 | 0.06-16 | 0.028 |
| AmpB (64-0.25) | 4-64 | 0.377 | 4->64 | 0.1505 |
Table 5: MIC range of antifungal drugs against 100 isolates of dermatophytes.
| Drugs | Trichophyton mentagrophytes species complex n=58 | Trichophyton rubrum n=42 | ||||
|---|---|---|---|---|---|---|
| Minimum range | Maximum range | Median (interquartile range) | Minimum range | Minimum range | Median (interquartile range) | |
| Amp B (64 μg/ml-0.25 μg/ml) | 2 | 64 | 4(4,8) | 2 | 64 | 4(4,8) |
| Fluconazole (64 μg/ml-0.25 μg/mll)) | 1 | 64 | 4(2,8) | 1 | 64 | 4(2,8) |
| Itraconazole (16 μg/ml-0.06 μg /ml)) | 0.06 | 64 | 0.06 | 0.06 | 16 | 0.06 |
| Voriconazole(32 μg/ml-0.125 μg/ml)) | 0.06 | 8 | 0.125 | 0.125 | 4 | 0.125 |
| Griseofulvin (16 μg/ml-0.06 μg/ml | 0.06 | 16 | 0.5(0.25,1) | 0.06 | 16 | 0.5(0.5,1) |
| Terbinafine (16 μg/ml-0.06 μg/ml)) | 0.06 | 8 | 0.1875(0.06,1) | 0.06 | 2 | 0.06(.06,1) |
| Sertaconazole (16 μg/ml-0.06 μg/ml) | 0.06 | 4 | 0.06(0.06,0.25) | 0.06 | 4 | 0.06(0.06,0.5) |
| Luliconazole(16 μg/ml-0.06 μg/ml) | 0.06 | 1 | 0.06 | 0.06 | 0.5 | 0.06 |
| Fenticonazole (16 μg/ml-0.06 μg/ml) | 0.06 | 1 | 0.06 | 0.06 | 0.5 | 0.06 |
| P value | <0.001 | <0.001 | ||||
Table 6: Minimum and maximum interquartile range of all the tested antifungals against Trichophyton mentagrophytes and Trichophyton rubrum.
Sensitive strains of terbinafine (60%) had MIC ranging from 0.06 to 0.25 μg/ml (Table 7). Among the 42 isolates of T. rubrum, 13 (30.9%) had higher MIC (range 0.5 μg/ml-2 μg/ml) whereas in T. mentagrophytes isolates, 26 out of 58 (44.8%) showed higher MIC range (0.5 μg/ml-8 μg/ml) for terbinafine.
| SL.NO. | Drugs | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | Range μg/ml | GM | MIC 50 | MIC 90 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Voriconazole (32 μg/ml-0.06 μg/ml) | 95 | 2 | 2 | 1 | 0.125- 4 | 0.1358 | 0.125 | 0.125 | |||||||
| 2 | Sertaconazole (16 μg/ml-0.06 μg/ml) | 76 | 4 | 11 | 5 | 2 | 2 | 0.06-4 | 0.099 | 0.06 | 0.06 | |||||
| 3 | Luliconazole (16 μg/ml-0.06 μg/ml) | 97 | 2 | 1 | 0.06 -1 | 0.07144 | <0.06 | <0.06 | ||||||||
| 4 | Fenticonazole (16 μg/ml-0.06 μg/ml) | 98 | 1 | 1 | 0.06 -1 | 0.07144 | <0.06 | <0.06 | ||||||||
| 5 | Griseofulvin (16 μg/ml-0.06 μg/ml | 7 | 6 | 14 | 44 | 22 | 3 | 2 | 2 | 0.06-16 | 2.004 | 0.5 | 1 | |||
| 6 | Terbinafine (16 μg/ml-0.06 μg/ml) | 55 | 5 | 11 | 23 | 5 | 1 | 0.06-8 | 0.1949 | 0.06 | 1 | |||||
| 7 | Fluconazole (64 μg/ml-0.25μg/ml) | 31 | 33 | 24 | 6 | 6 | 1-64 | 4.815 | 2 | 8 | ||||||
| 8 | Itraconazole (16 μg/ml-0.06 μg /ml) | 84 | 2 | 3 | 2 | 3 | 3 | 3 | 0.06-16 | 0.1112 | 0.06 | 0.06 | ||||
| 9 | Amp B (64 μg/ml-0.25μg/ml) |
- | - | - | - | - | 6 | 63 | 26 | 3 | 2 | 2- >64 | 4.975 | 4 | 4 |
Table 7: MIC distribution of Trichophyton spp. (n=100) against 9 antifungals drugs tested.
The MIC range of Griseofulvin for T. mentagrophytes was 0.06 μg/ml-4 μg/ml and for T. rubrum it was 0.125 μg/ml-16 μg/ml. Fifty two percent (22 out of 42) of T. rubrum and 26 (44.8%) isolates of T. mentagrophytes had an MIC of 0.5 μg/ml (limit of effectiveness). Among the azoles, both itraconazole (<0.06 μg/ ml) and voriconazole (0.125 μg/ml) had low MIC, except for two isolates of T. rubrum that had 16 μg/ml and 32 μg/ml. Luliconazole, fenticonazole and sertaconazole had a very low MIC value of 0.06 μg/ml. Fluconazole was less active with the MIC range of (2 μg/ml-64 μg/ml). Similarly, higher MIC range (4- >64 μg/ml) for amphotericin B was seen for all the isolates (Table 4). MICs for Trichophyton mentagrophytes ATCC 4439 were within the established range (Table 8).
| Dermatophyte | QC antifungal agent | MIC Range (μg/ml) |
|---|---|---|
| T. mentagrophytes | Griseofulvin | 0.125 -0.5 |
| ATCC MYA‑4439 | ||
| Itraconazole | 0.03-0.5 | |
| Terbinafine | 0.002-0.008 | |
| Voriconazole | 0.03-0.5 |
Table 8: Reference Minimum Inhibitory Concentration (MIC) range of antifungals used in the study (CLSI guidelines).
Friedman test was applied to Table 2. The analysis showed statistically significant difference between the drugs with respect to the concentration levels for both organisms with Pvalue <0.001. In multiple comparison, post hoc analysis with Wilcoxon signed rank test was conducted with Bonferroni correction and the significance level at P<0.005.
The mean MIC pattern of T. mentagrophytes and T. rubrum in our study in decreasing order was amphotericin B>fluconazole>griseofulvin>terbinafine>itraconazole>voriconaz ole>sertaconazole>luliconazole=fenticonazole.
Mannose Binding Lectin (MBL) by ELISA
Diluted samples that gave a mean absorbance of 0.5 ng/ml-40 ng/ml were in the detectable range of kit used in this study. Patients infected with dermatophytes had MBL levels from 7.21 ng/ml-31.79 ng/ml, while the healthy controls had values ranging from 0.048 ng/ml-0.107 ng/ml. Among the patients with MBL levels from 0.5 ng/ml to 10 ng/ml, majority (64.9%) had been infected by T. mentagrophytes, as compared to 13 (35.1%) patients with T. rubrum infection. Distribution of MBL levels in correlation with dermatophyte species and control group (n=100) is shown in Tables 9 and 10.
| Dermatophyte species | 0.5 ng/ml-10 ng/ml | 10 ng/ml-20 ng/ml | 20 ng/ml-30 ng/ml | 30 ng/ml-40 ng/ml |
|---|---|---|---|---|
| Trichophyton mentagrophyte (n=58) | 24 (41.4%) | 8 (13.8%) | 21 (36.2%) | 5 (8.6%) |
| Trichophyton rubrum (n=42) | 13 (30.9%) | 6 (14.3%) | 17 (40.5%) | 6 (14.3) |
Table 9: Distribution of mannose binding lectin range among the etiological agents.
| SL.NO | Group | MBL Mean | St. Deviation | P Value |
|---|---|---|---|---|
| 1 | Patients with dermatophytosis (n=100) | 17.73930 | 9.52119 | < 0.00 |
| 2 | Healthy individuals as controls (n=100) | 0.0677 | 0.14819 | < 0.00 |
Table 10: Levels of mannose binding Lectin in patients with dermatophytosis and healthy individuals.
Discussion
Patient in the age group of 30 years-40 years were most susceptible, followed by those in the age group of 20 years-30 years which was similar to the observation n of studies from north India [13,14]. Since patients in these age groups are actively involved in outdoor activities with increased sweating and may be with compromised maintenance of hygiene, chances of contracting dermatophyte infection increase. In recent years in India, there has been an epidemiological shift of dermatophytes causing infection of the skin from T. rubrum to T. mentagrophytes, which has emerged as the predominant organism. In this study, T. mentagrophytes complex (58%) was the most common organism isolated followed by T.rubrum (42%), similar to various studies conducted across India and Bangladesh [15-17]. In the past, T. rubrum was the most common etiological agent of chronic dermatophytosis [18-20]. In contrast to earlier studies, the present study revealed 56.2% of patients with chronic dermatophytosis being infected with T. mentagrophytes. The remaining 43.7% of patients had infection due to T. rubrum, which was in concordance with the study by Jamuna et al. (44.3%).
Biofilms enhance the virulence of the organism, increase the survival and act as a physical barrier against the host immune response, resulting in difficulty in treatment of the infection. While biofilm formation in onychomycosis has been documented, there is paucity of literature with regard to biofilm formation in dermatophytosis of the glabrous skin [21-23]. Orlandi CBC et al. reported that the biofilm produced by T. rubrum had increased density, and greater biomass and Extra cellular polysaccharide matrix (EPS) than that of T. mentagrophytes [20]. In this study, T. rubrum was observed to produce strong biofilm in more number of isolates (57.1%) than T. mentagrophytes. Similarly, among the organisms producing chronic dermatophytosis, strong biofilm was formed by 92.8% of T. rubrum as against 58.3% of T. mentagrophytes complex. Both these observations were of statistical significance. There is a need for large scale studies to document the significance and impact of biofilms produced by dermatophytes on the antifungal resistance and therapeutic outcome.
The MIC range of fluconazole and amphotericin B were similar for both T. mentagrophytes complex and T. rubrum. MIC was higher for griseofulvin (16 μg/ml for 2 isolates) and itraconazole (16 μg/ml for one isolate) against T. rubrum than T.mentagrophytes. On the other hand, MIC for terbinafine against T. mentagrophytes was higher than that of T. rubrum (MIC 8 μg/ml) for 2 isolates.
This was similar to the observation by Singh SK et al. [24] MIC of luliconazole and sertaconazole were in the susceptible range and were lower than the MIC range of these drugs in the study by Khurana et al. However, the MIC range of voriconazole was 0.125 μg/ml to 4 μg/ml as compared to 0.06 μg/ml to 2 μg/ml and 0.06 μg/ml to 16 μg/ml in other studies in north India [25]. In this study, it was observed that 89% of isolates were susceptible to itraconazole, 74% to griseofulvin, 64% to fluconazole and 60% to terbinafine. In the study done between 2014-2018 by Shaw et al, susceptibility percentage of the isolates for itraconazole, terbinafine fluconazole and griseofulvin was found to be 99.5%,72%, 67.4%, and 15.8% respectively [26]. This clearly highlights the difference in the susceptibility pattern of dermatophytes to griseofulvin in north and south India and further explains the clinical response to griseofulvin observed by dermatologists in this part of the country.
In the present study, terbinafine resistance was seen in 40% isolates. The MIC range of terbinafine varied from 0.06 μg/ml to >8 μg/ml in contrast to the studies from north India where the highest MIC was 32 μg/ml [22,24,25]. Another study had reported only one out of 64 isolates with MIC of 0.25 μg/ml contrary to 32 isolates with an MIC of 0.25 μg/ml in the present study. These findings are consistent with the observation by Ebert et al. who reported lower resistance rate of 16% in southern India as compared to 75% in north, west and eastern India [27-29].
Resistance to griseofulvin was found in 26% of strains, as they had a MIC of >0.5 μg/ml which is considered as the limit of effectiveness. Among the 74% of the isolates susceptible to griseofulvin, 46% had an MIC of 0.5 μg/ml. MIC range of griseofulvin ranged from 0.06 μg/ml to 16 μg/ml in contrast to the studies from north India which reported a higher range [26]. Out of the total isolates, 2 had MIC of 4 μg/ml and another 2 had MIC of 8 μg/ml.
MIC of fluconazole in the present study ranged from 1to 64 μg/ml for both T. mentagrophytes and T. rubrum. Similar observations were noticed in other studies, in which the MIC range of fluconazole for T. mentagrophytes varied from 0.06 μg/ml to >64 μg/ml [29-31]. In this study, 36 % isolates had high MIC in the range of 8 μg/ml-64 μg/ml which is similar to the observation of Shaw et al (32.5%) [26].
In the present study, MIC of itraconazole ranged from 0.06 μg/ml to >16 μg/ml which was similar to the report by Singh Sk et al. and Khurana A et al., while other studies had a narrow range of MIC (0.015-1;0.01 μg/ml-4 μg/ml) [24,25,31-33].
The MIC range of voriconazole (0.125 μg/ml-4 μg/ml) was found to correspond to the lower end of the MIC range observed in other studies (0.031 μg/ml-16 μg/ml, 0.06 μg/ml-16 μg/ml) [28,29] and higher than that reported by A. Khurana et al. (0.06 μg/ml-2 μg/ml) [25].
MIC of amphotericin B ranged from 4 μg/ml->64 μg/ml with resistance seen in 94% of strains as they had a MIC of >4 μg/ml (limit of effectiveness). The MIC range of this drug for both T. mentagrophytes and T. rubrum, is higher than that noted in previous studies [24-26] Out of all the drugs tested, amp B showed least sensitivity to dermatophytes.
MIC of sertaconazole was in the range of 0.06 μg/ml-4 μg/ml for T. mentagrophytes and 0.06 μg/ml for T. rubrum which is lesser than MIC range 0.25 μg/ml-16 μg/ml reported by a study from Delhi [25,34]. Sensitivity was seen in 91% of isolates with MIC of <0.5 μg/ml (limit of effectiveness). Luliconazole and fenticonazole exhibited the highets susceptibility for both T. mentagrophytes complex and T. rubrum with a MIC of 0.06 μg/ml. Although there are many studies with regard to susceptibility patterns offe nticonazole against superficial candidiasis, there is paucity of literature with regard to sensitivity against dermatophytes.
This study points to a rising proportion of strains of Trichophyton mentagrophytes with the change in the MIC pattern for terbinafine and griseofulvin which is slowly drifting towards increasing resistance reflecting the current pattern. Luliconazole, sertaconazole and fenticonazole showed good response. Current solution to the menace of dermatophytosis lies in the maintenance of skin hygiene, adherence to general measures, prudent use of antifungals, appropriate dosing and duration, compliance and performing antifungal susceptibility testing when feasible.
MBL in dermatophytosis
MBL deficiency hasb een considered ot predispose to recurrent skin infections [21]. The MBL and MASP (Mannose Binding Lectin-Associated Serine Proteases) seem to play a role in limiting the diseasbe y helping in the clearance of the apoptotic skin cells, promoting opsonophagocytictosis of the invading pathogens and hence the control of the spread of infection. Normal serum value of MBL has been mentioned as 800 ng/ml-1000 ng/ml there are no standard defined values to denote the deficiency of MBL. Values of 50, 100, 500 ng/ml or even 1000 ng/ml [36] has been considered as the cut off by researchers. It has also been observed that deficiency levels tend to vary in different diseases. In our study, both patients and the healthy controls had low levels of MBL, with the latter having much lower levels. Mean MBL among the patients and controls was 17.739 and 0.0677 respectively. In the study by Liu F et al [37]. on the role of MBL in patients with Vulvovaginal Candidiasis (VVC), the MBL concentration range was 0.01 ng/ ml-95.45 ng/ml among the patients with VVC and 0 ng/ml-4.01 ng/ml in controls. Median MBL concentration was 17.80 while among the controls it was 1.28. Authors had explained the higher levels of MBL as the result of the pro inflammatory action of this protein to help the host defense mechanism. As there is no data on levels of MBL in patients with dermatophytosis we are not able to compare our results. Hence to consider only the MBL levels as risk factors cannot be justified, as other factors also have to be looked into simultaneously, like defects in classical complement, humoral immunity or phagocytic pathway. Further studies need to be done to evaluate the role of MBL as a major risk factor for dermatophytosis [32-37].
Limitations
The sample size is too small to comment on the role of MBL. Other immunological parameters have to be considered for risk factors for dermatophytosis. The clinical response in patients, whose isolates were analyzed for in vitro sensitivity of the antifungals, was not evaluated.
Conclusion
Biofilm formation by dermatophytes contribute to therapeutic non- response causing challenge to clinicians. T. rubrum produced strong biofilm in higher proportion, especially in patients with chronic infection as compared to T. mentagrophytes complex. It was observed that in patients with first episode, none of the organisms produced strong biofilm. Further research on biofilm formation will facilitate newer therapeutic strategies. T.rubrum and T. mentagrophytes. Lowest MIC values were observed for fenticonazole and luliconazole. Among the systemic antifungals, the study isolates were found to be most susceptible to itraconazole followed by griseofulvin, against T. eb T. rubrun and T. mentagrophytes fluconazole and terbinafine. In the present scenario of increasing antifungal resistance, it is ideal to perform antifungal drug susceptibility tests in patients with chronic/recurrent/relapse dermatophytosis and treatment failure. As there are no clinical breakpoints defined as of yet, there is an urgent need to establish Epidemiological cut off values for dermatophytes and these values may be used by clinicians. Further large case control studies on MBL levels could throw light on predisposition to dermatophytosis and the treatment option of recombinant MBL protein.
References
- Dogra S, Uprety S (2016) The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J 7: 73-6.
[Crossref], [Google Scholar], [Indexed]
- Duek L, Kaufman G, Ulman Y, Berdicevsky I (2004) The pathogenesis of dermatophyte infections in human skin sections. J Infect 48: 175-180.
[Crossref], [Google Scholar], [Indexed]
- Ramaraj V, Vijayaraman R, Rangarajan S, Kindo A (2016) Incidence and prevalence of dermatophytosis in and around Chennai, Tamilnadu, India. Int J Res Med Sci 4: 695-700.
[Crossref], [Google Scholar]
- Hawser SP, Douglas LJ (1995) Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother 39: 2128-2131.
[Crossref], [Google Scholar], [Indexed]
- Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15: 167-193.
[Crossref], [Google Scholar], [Indexed]
- Marsh PD (2004) Dental plaque as a microbial biofilm. Caries Res 38: 204-211.
[Crossref], [Google Scholar], [Indexed]
- Eisen DP, Minchinton RM (2003) Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin Infect Dis 37: 1496-1505.
[Crossref], [Google Scholar], [Indexed]
- Miller C, Wilgenbusch S, Michael M, Chi DS, Youngberg G, et.al. (2010) Molecular defects in the mannose binding lectin pathway in dermatological disease: Case report and literature review. Clin Mol Allergy 8: 6.
[Crossref], [Google Scholar], [Indexed]
- Falahati M, Nami S, Zeini F, Ghelman M, Ghasemi Z, et.al. (2013) Investigation of Mannose–Binding Lectin Level and Deficiency in Patients with Dermatophytosis. Jundishapur J Microbiol 6: e94140.
[Crossref], [Google Scholar]
- Dommett RM, Klein N, Turner MW (2006) Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens 68: 193-209.
[Crossref], [Google Scholar], [Indexed]
- Auriti C, Prencipe G, Moriondo M, Bersani I, Bertaina C, et.al. (2017) Mannose-Binding Lectin: Biologic Characteristics and Role in the Susceptibility to Infections and Ischemia-Reperfusion Related Injury in Critically Ill Neonates. J Immunol Res 1-11.
[Crossref], [Google Scholar], [Indexed]
- Rengasamy M, Shenoy MM, Dogra S, Asokan N, Khurana A, et.al. (2020) Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) Task Force against Recalcitrant Tinea (ITART) Consensus on the Management of Glabrous Tinea (INTACT). Indian Dermatol Online J 11: 502-519.
[Crossref], [Google Scholar], [Indexed]
- Verma S, Verma G, Sharma V, Bhagra s, Negi A, et.al. (2017) Current Spectrum of Dermatophytosis in a Tertiary Care Hospital of North India – A 6-Year Clinico-Mycological Study. J Med Sci Clin Res 5: 19488-19494.
[Crossref], [Google Scholar]
- Gupta SK, Prasad J, Brahmane R B (2017) Clinico-mycological study of Dermatophytosis at a tertiary medical center of Uttar Pradesh. Trop J Pathol Microbiol 3: 283-288.
[Crossref], [Google Scholar]
- Bhatia VK, Sharma PC (2014) Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. Springerplus 3: 134.
[Crossref], [Google Scholar], [Indexed]
- Jamuna SL, Kaviarasan PK, Prasad PV, kanmabal K, Poorna B, et.al. (2019) Menace of Chronic Dermatophytosis - A Descriptive Study in a Tertiary Care Center. J Med Sci Clin Res 7: 128-133.
[Crossref], [Google Scholar]
- Khan SA, Shamsuzzaman SM, Rahman AK, Asekin NA, Mahmud R, et.al. (2021) Isolation and Identification of Dermatophytes Causing Dermatophytosis at a Tertiary Care Hospital in Bangladesh. Arch Clin Biomed Res 5: 437-451.
- Hay RJ, Brostoff J (1977) Immune responses in patients with chronic Trichophyton rubrum infections. Clin Exp Dermatol 2: 373–380.
[Crossref], [Google Scholar], [Indexed]
- Sentamilselvi G, Janaki C, Ajitha D, Kamalam A, Thambiah AS (2000) Chronic Dermatophytosis Indian J Dermatol 45: 168-173.
[Crossref]
- Gupta A (2012) MBL deficiency as risk of infection and autoimmunity. Animal Lectins: Form, Function and Clinical Applications : 933-953.
- Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, et.al. (2021) The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol 87: 468-82.
[Crossref], [Google Scholar], [Indexed]
- Costa-Orlandi CB, Sardi JCO, Santos CT, Fusco-Almeida AM, Mendes-Giannini MJS et.al. (2014) In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms, Biofouling: The Journal of Bioadhesion and Biofilm Research 30: 719-727.
[Crossref], [Google Scholar], [Indexed]
- Singh SK, Patwa DK, Tilak R, Das A, Singh TB, et.al. (2019) susceptibility of dermatophytes to oral antifungal drugs and amphotericin B in Uttar Pradesh, India. Indian J Dermatol Venereol Leprol 85: 388-392.
[Crossref], [Google Scholar], [Indexed]
- Khurana A, Masih A, Chowdhary A, Sardana K, Borker S, et.al. (2018) Correlation of Susceptibility Based on MICs and Squalene Epoxidase Mutations with Clinical Response to Terbinafine in Patients with Tinea Corporis/Cruris. Antimicrob Agents Chemother 62: e01038- e01018.
[Crossref], [Google Scholar], [Indexed]
- Shaw D, Singh S, Dogra S, Jayaraman J, Bhat R, et.al. (2020) Minimal inhibitory concentration and upper limit of wild type distribution for 13 antifungal agents against Trichophyton mentagrophytes/interdigitale complex of Indian origin. Antimicrob Agents Chemother 64: e01964- e01919.
[Crossref], [Google Scholar], [Indexed]
- CLSI (2008) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved standard-2nd ed.
- 28.Singh A, Masih A, Khurana A, Singh PK, Gupta M, et.al. (2018) High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 61: 477-484.
[Crossref], [Google Scholar], [Indexed]
- Ebert A, Monod M, Salamin K, Burmester A, Uhrla S, et.al. (2020) Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentric study. Mycoses 63: 717-728.
[Crossref], [Google Scholar], [Indexed]
- Silva LB, de Oliveira DBC, da Silva BV, De Souza RA, Da Silva PR, et.al. (2014) Identification and antifungal susceptibility of fungi isolated from dermatomycoses. J Eur Acad Dermatol Venereol 28: 633-640.
[Crossref], [Google Scholar], [Indexed]
- Pathania S, Rudrmurthy SM, Narang T, Saikia UN, Dogra S, et.al. (2018) A Prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol,Venereol & Leprol 84: 678-684.
[Crossref], [Google Scholar], [Indexed]
- Fernández-Torres B, Carrillo AJ, Martı́n E, Del palacio A, Moore MK, et.al. (2001) In Vitro Activities of 10 Antifungal Drugs against 508 Dermatophyte Strains. Antimicrob Agents Chemother 45: 2524-2528.
[Crossref], [Google Scholar], [Indexed]
- Singh J, Zaman M, Gupta AK (2007) Evaluation of microdilution and disk diffusion methods for antifungal susceptibility testing of dermatophytes. Med Mycol 45: 595-602.
[Crossref], [Google Scholar], [Indexed]
- Deng S, Zhang C, Seyedmousavi S, Zhu S, Tan X, et.al. (2015) Comparison of the in vitro activities of newer triazoles and established antifungal agents against Trichophyton rubrum. Antimicrob Agents Chemother 59: 4312-4314.
[Crossref], [Google Scholar], [Indexed]
- Magagnin CM, Stopiglia CDO, Vieira FJ, Heidrich D, Machado M, et.al. (2011) Antifungal susceptibility of dermatophytes isolated from patients with chronic renal failure. An Bras Dermatol 86: 694-701.
[Crossref], [Google Scholar], [Indexed]
- Singh A, Masih A, Monroy-Nieto J, Singh PK, Bowers J, et.al. (2019) A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: Genomic insights and resistance profile. Fungal Genet Biol 133: 103266.
[Crossref], [Google Scholar], [Indexed]
- Mutcali S, Saltoglu N, Balkan II, Ozaras R, Yemisen M, et.al. (2016) Early Changes of Mannose-Binding Lectin, H-Ficolin, and Procalcitonin in Patients with Febrile Neutropenia: A Prospective Observational Study. Turk J Hematol 33: 304-310.
[Crossref], [Google Scholar], [Indexed]
- Liu F, Liao Q, Liu Z (2006) Mannose-binding lectin and vulvovaginal candidiasis. International Journal of Gynecology & Obstetrics 92: 43-7.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences